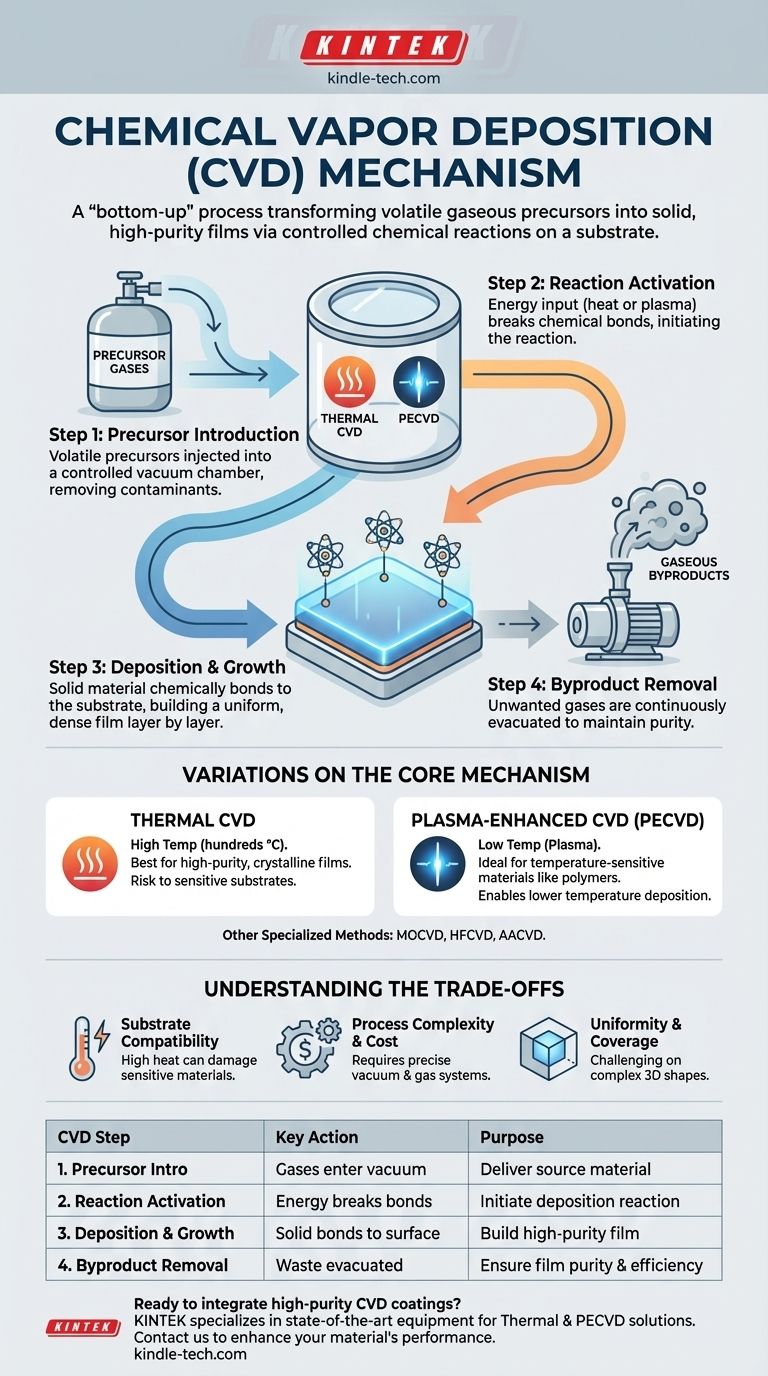
At its core, the mechanism of Chemical Vapor Deposition (CVD) is a process where volatile chemical precursors in a gaseous state are transformed into a solid, high-purity film on the surface of a substrate. This transformation is triggered by a controlled chemical reaction within a vacuum chamber, causing the desired material to deposit and build up layer by layer, chemically bonding to the surface.
Chemical Vapor Deposition is not simply spraying a material onto a surface; it is a "bottom-up" manufacturing technique that builds a solid material directly from its chemical components in a gas phase. The core mechanism relies on inducing a chemical reaction that forces these gas precursors to solidify onto a target.

The CVD Mechanism: A Step-by-Step Breakdown
To understand how CVD works, it is best to break the process down into its fundamental stages. Each step is critical for controlling the quality, thickness, and properties of the final deposited film.
Step 1: Introducing the Precursor
The process begins with one or more volatile chemicals, known as precursors. These are compounds that contain the elements you wish to deposit.
These precursors are injected as a gas into a sealed reaction chamber, which is held under a controlled vacuum. The vacuum is essential for removing air and other contaminants that could interfere with the reaction or be incorporated as impurities in the final film.
Step 2: Activating the Reaction
Once inside the chamber, the precursor gases need an input of energy to initiate the chemical reaction. This energy breaks the chemical bonds within the precursor molecules.
The most common method is applying heat, a process known as Thermal CVD. The entire chamber, including the substrate material, is heated to a specific temperature that causes the precursors to either decompose or react with other gases.
Step 3: Deposition and Film Growth
As the precursor gases react or decompose, they form a non-volatile solid. These newly formed solid particles are then deposited onto the surface of the substrate (the workpiece being coated).
The material doesn't just "stick" to the surface; it forms strong chemical bonds. This results in a dense, strongly adhered film that grows uniformly over the entire exposed surface, one atomic or molecular layer at a time.
Step 4: Removing the Byproducts
The chemical reaction almost always produces unwanted gaseous byproducts in addition to the desired solid material.
These waste gases are continuously removed from the chamber by the vacuum system, preventing them from contaminating the film and ensuring the deposition reaction continues efficiently.
Variations on the Core Mechanism
The method used to provide the activation energy in Step 2 defines the different types of CVD. The choice of method depends on the desired film properties and the temperature sensitivity of the substrate.
Thermal CVD
This is the classic approach, relying on high temperatures (often several hundred to over a thousand degrees Celsius) to drive the reaction. It is effective for creating very high-purity, crystalline films.
Plasma-Enhanced CVD (PECVD)
Instead of high heat, PECVD uses a plasma (an ionized gas) to energize the precursor gases. The highly reactive ions and electrons in the plasma can break down precursor molecules at much lower temperatures.
This makes PECVD ideal for depositing films onto substrates that cannot withstand the high heat of thermal CVD, such as plastics or certain electronic components.
Other Specialized Methods
Other variants exist to meet specific needs. Metal-Organic CVD (MOCVD) uses metal-organic precursors, common in semiconductor manufacturing. Hot-Filament CVD (HFCVD) uses a heated wire to catalytically decompose the precursors, while Aerosol-Assisted CVD (AACVD) delivers the precursor via an aerosol spray.
Understanding the Trade-offs
While powerful, the CVD mechanism is not without its challenges. Understanding its limitations is key to using it effectively.
Substrate Compatibility
The high temperatures required for traditional Thermal CVD can damage or destroy heat-sensitive substrates. This is the primary driver for using lower-temperature alternatives like PECVD, even if it sometimes results in a slightly lower-quality film.
Process Complexity and Cost
CVD is a high-precision process that requires expensive vacuum chambers, gas delivery systems, and control electronics. The precursor chemicals themselves can also be costly, toxic, or difficult to handle safely.
Uniformity and Coverage
While CVD is known for producing uniform coatings, ensuring that uniformity across complex, three-dimensional shapes can be challenging. Gas flow dynamics and temperature gradients within the chamber must be carefully managed.
How to Apply This to Your Project
The specific CVD mechanism you choose should be dictated by the primary goal for your material or component.
- If your primary focus is ultimate purity and film quality: Thermal CVD is often the superior choice, as the high temperatures enable the growth of highly ordered, low-defect films, which is why it's a leading method for producing high-performance graphene.
- If your primary focus is coating a temperature-sensitive material: Plasma-Enhanced CVD (PECVD) is the necessary approach, as it allows deposition to occur at temperatures low enough to protect materials like polymers or pre-existing electronics.
- If your primary focus is enhancing surface durability: Any CVD method can work, as the key benefit is the strong chemical bond that creates a much more robust coating than a simple physical deposition process.
Ultimately, the CVD mechanism is a versatile and foundational tool for engineering materials at the atomic scale.
Summary Table:
| CVD Step | Key Action | Purpose |
|---|---|---|
| 1. Precursor Introduction | Volatile gases enter a vacuum chamber. | Deliver source material for the film. |
| 2. Reaction Activation | Energy (heat, plasma) breaks chemical bonds. | Initiate the deposition reaction. |
| 3. Deposition & Growth | Solid material bonds to the substrate surface. | Build a high-purity, adherent film layer by layer. |
| 4. Byproduct Removal | Gaseous waste is evacuated by the vacuum system. | Ensure film purity and process efficiency. |
Ready to integrate high-purity CVD coatings into your laboratory processes?
At KINTEK, we specialize in providing state-of-the-art lab equipment and consumables for all your deposition needs. Whether you require the ultimate purity of Thermal CVD or the versatility of Plasma-Enhanced CVD (PECVD) for temperature-sensitive substrates, our solutions are designed to deliver robust, uniform films with strong chemical adhesion.
Let our experts help you select the perfect CVD mechanism for your project. Contact KINTEK today to discuss how our equipment can enhance your material's performance and durability.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What role does CVD equipment play in single-layer graphene-coated metal catalysts? Unlock High-Performance Stability
- What is DC sputtering of metals? A Simple, Fast Method for High-Quality Metal Films
- What is CVD with example? A Guide to the Process Behind Lab-Grown Diamonds & Microchips
- What is the application of DC sputtering? A Guide to Cost-Effective Metal Coating
- What are the two techniques used for preparing nano thin films? A Guide to PVD and CVD Methods
- What are the methods of deposition of thin films? A Guide to PVD, CVD, and ALD Techniques
- What role does a resistance heating furnace play in CVD tantalum coating? Master Thermal Precision in CVD Systems
- What is a major limitation of standard CVD? Solve the Thermal Barrier with Advanced Coating Solutions



















