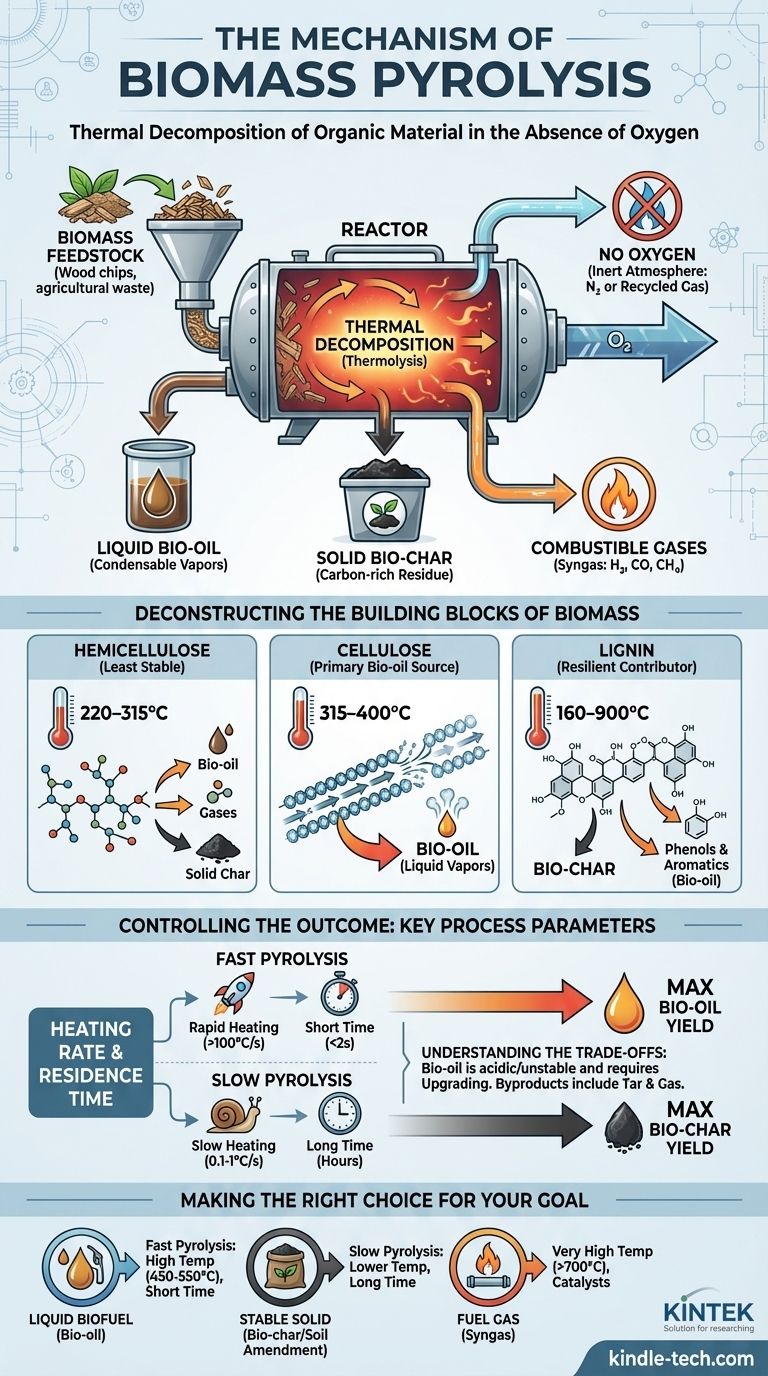
At its core, biomass pyrolysis is the thermal decomposition of organic material in the complete absence of oxygen. The process breaks down the complex polymers in biomass—primarily cellulose, hemicellulose, and lignin—into a mixture of liquid bio-oil, solid bio-char, and combustible gases by carefully controlling heat and time.
Pyrolysis is not burning; it is a controlled deconstruction process. By preventing combustion, high heat is used to systematically fracture the chemical bonds within biomass, allowing us to capture valuable liquid, solid, and gaseous products instead of just releasing energy as heat and light.

The Core Principle: Thermal Decomposition Without Oxygen
What is Thermolysis?
Pyrolysis is a specific type of thermolysis, which simply means "breakdown by heat." In an oxygen-rich environment, heat would cause the biomass to combust, or burn.
By removing oxygen, we prevent combustion. Instead, the intense vibration from the high heat energy forces the large organic polymers to crack and break apart into smaller, more volatile molecules.
The Critical Role of an Inert Atmosphere
The process is conducted in an inert (non-reactive) atmosphere, often using recycled pyrolysis gas or nitrogen. This ensures that the valuable smaller molecules, once created, are not immediately oxidized (burned). Instead, they are carried out of the reactor to be condensed and collected.
Deconstructing the Building Blocks of Biomass
Biomass is not a single substance. Its behavior during pyrolysis is dictated by the thermal stability of its three main components.
Hemicellulose: The First to Break
Hemicellulose is the least stable component, decomposing at relatively low temperatures, typically between 220–315°C.
Its decomposition is complex, yielding some volatile liquids (bio-oil), non-condensable gases, and a significant amount of solid char.
Cellulose: The Primary Source of Bio-oil
Cellulose is more thermally stable due to its crystalline structure, decomposing rapidly over a narrow and higher temperature range of 315–400°C.
The rapid "unzipping" of cellulose polymers is the primary pathway for producing high yields of liquid vapors, which are then condensed into bio-oil. Maximizing this reaction is the goal of fast pyrolysis for biofuel production.
Lignin: The Resilient Contributor to Bio-char
Lignin is a highly complex, aromatic polymer that is very difficult to break down. It decomposes slowly across a very broad temperature range, from 160°C up to 900°C.
Because it doesn't easily vaporize, lignin primarily contributes to the formation of bio-char. It also yields phenols and other complex aromatic compounds found in bio-oil.
Controlling the Outcome: Key Process Parameters
The final product yields can be precisely manipulated by adjusting the conditions of the pyrolysis process.
The Impact of Heating Rate
Fast pyrolysis, characterized by very rapid heating, is designed to maximize the liquid yield. It heats the biomass so quickly that the cellulose and hemicellulose vaporize before they have a chance to undergo secondary reactions that form more char and gas.
Slow pyrolysis, which involves slow heating over hours, allows for these secondary reactions to occur. This process maximizes the yield of bio-char.
The Importance of Residence Time
Residence time refers to how long the material stays in the hot reactor. For fast pyrolysis, a short vapor residence time (typically less than 2 seconds) is critical.
This quickly removes the hot vapors from the reactor before they can crack further into low-value gases, preserving the molecular structures that form the liquid bio-oil upon cooling.
Understanding the Trade-offs
While powerful, pyrolysis is not a perfect process. Understanding its inherent challenges is key to successful application.
The Challenge of Product Quality
The raw liquid product, often called bio-oil or pyrolysis oil, is not a drop-in replacement for petroleum fuels. It is acidic, corrosive, chemically unstable, and contains a significant amount of water and oxygenated compounds.
This means it requires significant and often costly upgrading and refining before it can be used as a transportation fuel.
The Problem of Byproducts
The process inevitably produces non-condensable gases and tar, a complex mixture of heavy organic compounds. Tars can clog equipment and reduce the efficiency of the process.
Likewise, the bio-char produced contains ash and must be managed. While it can be a valuable product, it can also be a waste stream if no market exists for it.
Making the Right Choice for Your Goal
The optimal pyrolysis strategy depends entirely on your desired end product.
- If your primary focus is producing liquid biofuel (bio-oil): Employ fast pyrolysis with high temperatures (450-550°C) and very short vapor residence times to maximize the breakdown of cellulose into condensable vapors.
- If your primary focus is producing a stable solid (bio-char) for soil amendment or carbon sequestration: Use slow pyrolysis with lower temperatures and long residence times to maximize char formation from all components.
- If your primary focus is generating fuel gas (syngas): Utilize very high temperatures (>700°C) and potentially catalysts to encourage the secondary cracking of all vapors into permanent gases like hydrogen, carbon monoxide, and methane.
Understanding these fundamental mechanisms empowers you to engineer a process that transforms raw biomass into a targeted, valuable resource.
Summary Table:
| Process Parameter | Impact on Product Yield | Typical Conditions |
|---|---|---|
| Heating Rate | Fast Pyrolysis: Maximizes Bio-oil Slow Pyrolysis: Maximizes Bio-char |
Fast: >100°C/s Slow: 0.1-1°C/s |
| Temperature | Lower (<400°C): More Char Higher (450-700°C): More Oil/Gas |
300-700°C |
| Residence Time | Short (<2s): Maximizes Bio-oil Long (hours): Maximizes Bio-char |
Fast: <2s (vapor) Slow: 30+ min (solid) |
| Biomass Component | Cellulose: Primary Bio-oil source Lignin: Primary Bio-char source |
Cellulose decomposes 315-400°C Lignin decomposes 160-900°C |
Ready to engineer your biomass pyrolysis process for maximum yield of bio-oil, bio-char, or syngas? KINTEK specializes in high-quality lab equipment and consumables for pyrolysis research and development. Our reactors, temperature controllers, and analytical tools are designed to help you precisely control heating rates, temperatures, and residence times—empowering you to optimize your process for your target product. Let our experts help you select the right equipment for your laboratory's specific biomass conversion goals.
Contact KINTEK today to discuss your pyrolysis application and discover the right solutions for your lab.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Mesh belt controlled atmosphere furnace
People Also Ask
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas













