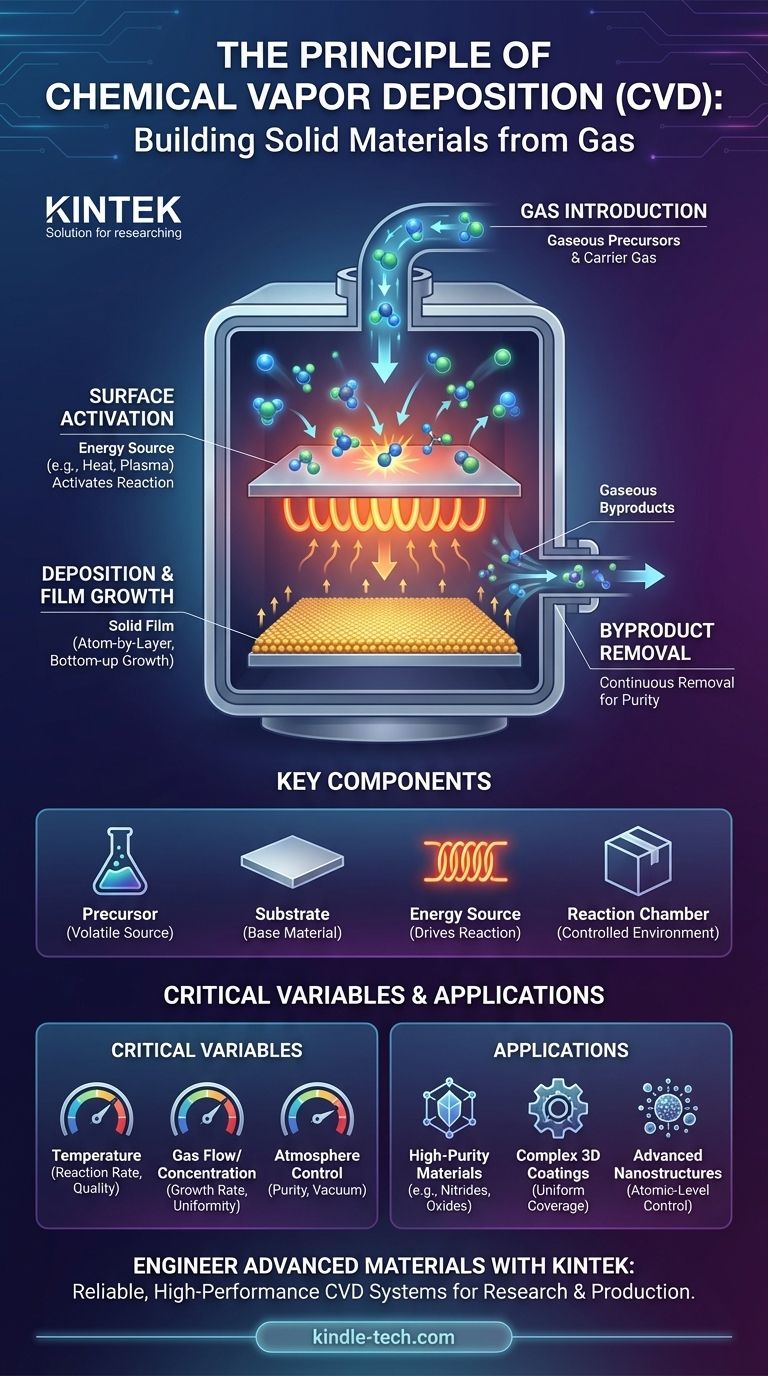
At its core, Chemical Vapor Deposition (CVD) is a process of building a solid material layer from a gas. It is founded on the principle of a controlled chemical reaction. Gaseous chemical precursors are introduced into a chamber where they react or decompose on a heated surface—the substrate—depositing a thin, non-volatile solid film onto it while gaseous byproducts are carried away.
The fundamental principle of CVD is a controlled chemical transformation, not a simple physical change. Instead of merely condensing a vapor onto a surface, CVD uses energy, typically heat, to break down precursor gases and form a completely new, solid material directly onto the substrate.

The CVD Process: A Step-by-Step Breakdown
To fully grasp the principle, it's helpful to visualize the process as a sequence of distinct events occurring within a specialized reactor. Each step is critical for the successful growth of the final film.
1. Introduction of Reactant Gases
The process begins by introducing one or more volatile precursor gases into the reaction chamber. These gases contain the specific elements that are intended to form the final solid film. Often, a carrier gas is used to transport the precursors to the substrate.
2. Activation on the Substrate Surface
The substrate is heated to a precise, often elevated, temperature. This heat provides the necessary thermal energy to activate the chemical reaction, causing the precursor gases to decompose or react when they come into contact with the hot surface.
3. Deposition and Film Growth
As the precursor gases react on the substrate, a stable solid product is formed. This solid material adheres to the surface, creating a thin film. The process is "bottom-up," meaning the film grows atom by atom or layer by layer, leading to a highly controlled structure.
4. Removal of Byproducts
The chemical reaction that forms the solid film also generates unwanted gaseous byproducts. These byproducts are removed from the reaction chamber by a continuous gas flow, ensuring the purity of the deposited film.
Key Components of the System
The CVD principle is realized through the interaction of a few core components. Understanding their roles clarifies how the process is controlled.
The Precursor
This is the volatile chemical compound that serves as the source for the desired film. The choice of precursor is critical as it dictates the composition of the final material and the required reaction conditions (like temperature).
The Substrate
This is the material or object onto which the thin film is grown. Its surface acts as the catalyst and foundation for the chemical reaction and deposition.
The Energy Source
Energy is required to drive the chemical reaction. While high heat is the most common method, other sources like plasma can also be used in variants like Plasma-Enhanced CVD (PECVD) to achieve reactions at lower temperatures.
The Reaction Chamber
This is the sealed, atmosphere-controlled environment where the entire process takes place. It allows for precise control over temperature, pressure, and gas flow, which are essential for creating a high-quality, uniform film.
Understanding the Key Variables
The success of the CVD process hinges on precise control. Mismanaging these variables can lead to poor film quality, lack of uniformity, or complete process failure.
Temperature is Paramount
The substrate temperature is one of the most critical parameters. It directly influences the reaction rate and the structural quality (crystallinity) of the resulting film. Too low, and the reaction won't occur; too high, and unwanted side reactions can take place.
Gas Flow and Concentration
The rate at which precursor gases are introduced and byproducts are removed affects the film's growth rate and uniformity. The concentration of the reactants must be carefully managed to ensure a stable, repeatable process.
Atmosphere Control is Non-Negotiable
CVD must be conducted in a highly controlled atmosphere or vacuum. Any impurities, such as oxygen or water vapor, can lead to contamination and defects in the final film, compromising its performance.
Applying This Principle to Your Goal
The choice to use CVD is typically driven by the need for high-performance materials with specific properties. Your goal will determine how you leverage the process.
- If your primary focus is creating extremely pure, high-performance materials: CVD is an exceptional choice for depositing inorganic materials like nitrides, carbides, and oxides with excellent density and quality.
- If your primary focus is coating complex, three-dimensional shapes: The gaseous nature of the precursors allows them to penetrate and uniformly coat intricate surfaces that are inaccessible to line-of-sight deposition methods.
- If your primary focus is developing advanced nanostructures: The "bottom-up" growth mechanism of CVD provides the atomic-level control necessary for synthesizing thin films and nanoparticles with precise characteristics.
By understanding that CVD is fundamentally a process of chemical creation on a surface, you can effectively harness it to engineer advanced materials from the ground up.
Summary Table:
| CVD Process Step | Key Function | Critical Variables |
|---|---|---|
| 1. Gas Introduction | Introduce precursor gases into the chamber. | Gas concentration, flow rate. |
| 2. Surface Activation | Heat substrate to drive the chemical reaction. | Substrate temperature. |
| 3. Film Deposition | Solid film grows atom-by-layer on the substrate. | Reaction rate, film uniformity. |
| 4. Byproduct Removal | Evacuate gaseous byproducts from the chamber. | Pressure, gas flow. |
Ready to Engineer Advanced Materials with Precision?
Understanding the principle of CVD is the first step. Implementing it successfully requires reliable, high-performance equipment. KINTEK specializes in providing laboratory-grade CVD systems and consumables tailored to your research and production goals.
Whether you are developing high-purity coatings, uniform 3D surface treatments, or advanced nanostructures, our expertise ensures you have the control and purity needed for success.
Contact KINTEK today to discuss how our CVD solutions can bring your material science projects to life.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- How does an HDP-CVD reaction chamber function? Master Dual-RF Control for Superior Gap Filling
- What is a primary challenge in producing usable graphene sheets after CVD? Overcoming the Transfer Bottleneck
- Which synthesis method is used for preparation of nanotubes? Master Scalable Production with CVD
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- How is magnetron sputtering different from other methods? Unlock High-Speed, Quality Thin Films
- Why is film thickness important? It's the key to controlling material performance.
- What is the inert gas used in sputtering? Maximize Your Thin-Film Deposition Efficiency
- What temperature is maintained in CVD? Unlocking the High-Heat Process for Superior Coatings



















