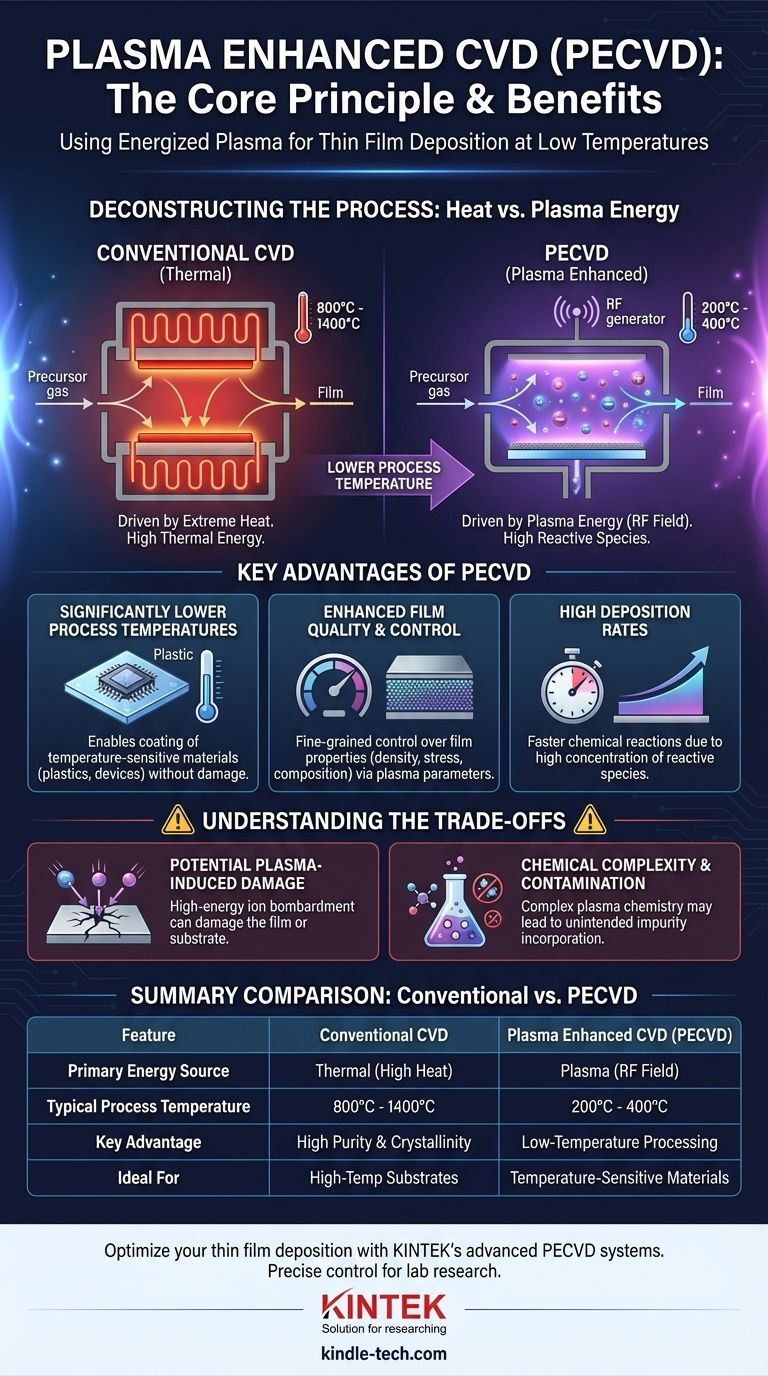
The core principle of Plasma Enhanced Chemical Vapor Deposition (PECVD) is the use of an energized plasma to decompose precursor gases into reactive molecules at significantly lower temperatures than required by conventional Chemical Vapor Deposition (CVD). This plasma, generated typically by a radio frequency (RF) field, provides the necessary energy for chemical reactions, allowing a thin film to form on a substrate without relying on extreme heat.
PECVD fundamentally changes how deposition energy is delivered. Instead of using brute-force thermal energy to break chemical bonds, it uses the targeted electrical energy of a plasma, enabling high-quality film growth on materials that cannot withstand high temperatures.

Deconstructing the PECVD Process
To understand PECVD, it is essential to first grasp the principles of conventional CVD and then see how the addition of plasma transforms the process.
The Foundation: Conventional CVD
Traditional Chemical Vapor Deposition is a process driven by heat. One or more volatile precursor gases are introduced into a reaction chamber containing a heated substrate.
At very high temperatures, typically ranging from 800°C to over 1400°C, the precursor gases have enough thermal energy to chemically react or decompose on or near the hot substrate surface.
This reaction results in the formation of a solid material, which is deposited as a thin, uniform film onto the substrate. The remaining gaseous byproducts are then exhausted from the chamber.
The "Plasma Enhanced" Innovation
PECVD introduces a critical new element: plasma. A plasma is a state of matter where a gas is energized to the point that its atoms are ionized, creating a mixture of ions, electrons, and highly reactive neutral molecules called radicals.
In a PECVD system, this is achieved by applying a strong electromagnetic field, usually radio frequency (RF), to the low-pressure gas inside the chamber.
How Plasma Replaces Extreme Heat
The key to PECVD is that the particles within the plasma are extremely reactive. These radicals and ions are chemically unstable and eager to react to form more stable compounds.
This high reactivity means they no longer need immense thermal energy to initiate the deposition reaction. The energy has already been supplied by the plasma field to create them.
As a result, the substrate can be kept at a much lower temperature (often 200°C to 400°C) while the chemical reactions still proceed efficiently, driven by the reactive species generated in the plasma.
Key Advantages of Using Plasma
Introducing plasma is not just an alternative; it provides distinct and powerful advantages that expand the applications of thin film deposition.
Significantly Lower Process Temperatures
This is the most critical benefit of PECVD. The ability to deposit films at lower temperatures makes it possible to coat temperature-sensitive substrates, such as plastics, polymers, or fully fabricated semiconductor devices with delicate integrated circuits. These materials would be damaged or destroyed by the high heat of conventional CVD.
Enhanced Film Quality and Control
The plasma's energy and density can be precisely controlled by adjusting the RF power and gas pressure. This gives engineers fine-grained control over the deposition rate and the final properties of the film, such as its density, stress, and chemical composition.
High Deposition Rates
Because the plasma creates a high concentration of reactive species, the chemical reactions can occur more rapidly than in many thermal CVD processes. This allows for faster film growth, which is a significant advantage in manufacturing environments.
Understanding the Trade-offs
While powerful, PECVD is not without its challenges. An objective assessment requires acknowledging its limitations.
Potential for Plasma-Induced Damage
The high-energy ions within the plasma can bombard the substrate surface during deposition. This bombardment can sometimes cause structural damage to the growing film or the underlying substrate, which is a concern in applications like advanced microelectronics.
Chemical Complexity and Contamination
Plasma chemistry is incredibly complex. The precursor gases can break down into many different species, not all of which are desirable. For instance, in silicon nitride deposition, hydrogen from the precursors can be incorporated into the final film, altering its electrical properties.
Equipment Complexity
A PECVD reactor, with its RF power source, vacuum systems, and control electronics, is more complex and generally more expensive than a simple thermal CVD furnace. This adds to both the capital and maintenance costs of the process.
Making the Right Choice for Your Goal
Selecting the right deposition method depends entirely on the material constraints and desired outcome of your project.
- If your primary focus is depositing films on temperature-sensitive materials: PECVD is the definitive and often only choice due to its low-temperature operation.
- If your primary focus is achieving the highest possible film purity and crystallinity: Conventional high-temperature CVD may be superior, as the thermal energy helps anneal defects and drive off impurities.
- If your primary focus is versatility and control over film properties: PECVD offers a wider process window, allowing you to tune film characteristics like stress and refractive index by adjusting plasma parameters.
Ultimately, PECVD leverages plasma physics to overcome the thermal limitations of traditional deposition, opening up new possibilities in material science and engineering.
Summary Table:
| Feature | Conventional CVD | Plasma Enhanced CVD (PECVD) |
|---|---|---|
| Primary Energy Source | Thermal (High Heat) | Plasma (RF Field) |
| Typical Process Temperature | 800°C - 1400°C | 200°C - 400°C |
| Key Advantage | High Purity & Crystallinity | Low-Temperature Processing |
| Ideal For | High-Temperature Substrates | Temperature-Sensitive Materials (e.g., plastics, semiconductors) |
Need to deposit high-quality thin films on temperature-sensitive materials? KINTEK specializes in advanced lab equipment, including PECVD systems, to meet your specific research and production needs. Our solutions enable precise control over film properties for applications in semiconductors, optics, and more. Contact our experts today to discuss how we can enhance your laboratory capabilities!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition



















