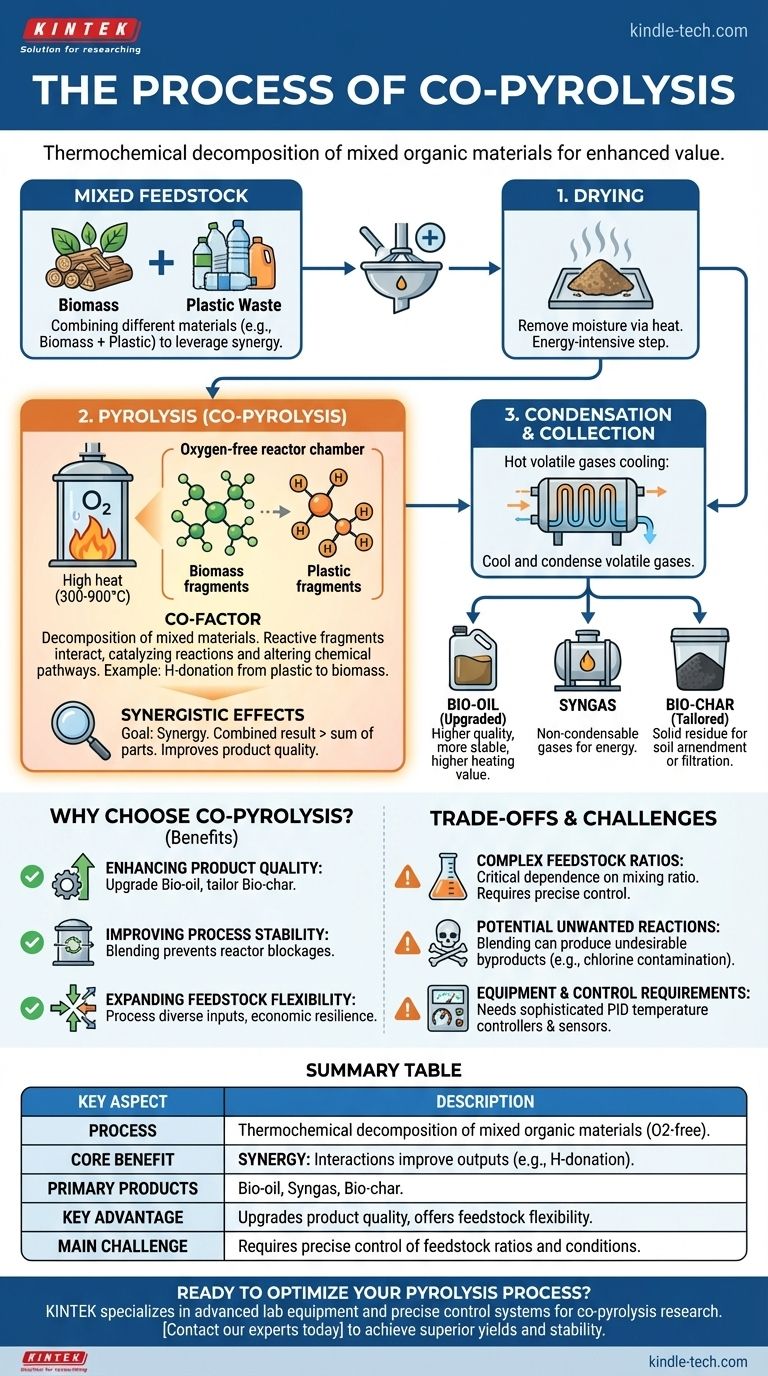
In essence, co-pyrolysis is the thermochemical decomposition of a mixture of two or more different organic materials at high temperatures in an oxygen-free environment. While standard pyrolysis processes a single type of feedstock, co-pyrolysis deliberately combines different materials—such as biomass with plastic waste—to leverage synergistic effects and improve the final product quality or process efficiency.
The critical distinction is not the process itself, but the strategy. Co-pyrolysis uses the same fundamental principles as pyrolysis but applies them to a mixed feedstock to overcome the limitations of a single material and create more valuable outputs.

Deconstructing the Process: From Pyrolysis to Co-Pyrolysis
To understand co-pyrolysis, you must first grasp the foundational mechanics of pyrolysis. The process is the same, but the interactions between the feedstocks are what make co-pyrolysis a distinct and powerful technique.
The Foundation: The Three Stages of Pyrolysis
Any pyrolysis process, whether with a single feedstock or a mixture, follows three core stages:
- Drying: The raw material is heated to remove residual moisture. This is a crucial, energy-intensive step that prepares the material for thermal decomposition.
- Pyrolysis: In an oxygen-starved chamber, the dried feedstock is heated to high temperatures (typically 300-900°C). The intense heat breaks down the complex chemical bonds, creating a mix of volatile gases and solid residue.
- Condensation & Collection: The hot volatile gases are cooled and condensed into a liquid known as bio-oil. The non-condensable gases (syngas) and the remaining solid residue (bio-char) are collected separately.
The "Co-" Factor: What Changes with a Mixture?
In co-pyrolysis, these same three stages occur. The key difference happens during the second stage, where the different materials decompose together. This is not just a simple average of the two materials' outputs.
Instead, reactive fragments from one material interact with fragments from the other. This interaction can catalyze reactions, donate components like hydrogen, and fundamentally alter the chemical pathways of decomposition.
Synergistic Effects: The Core Benefit
The primary goal of co-pyrolysis is to achieve synergy, where the combined result is greater than the sum of its parts.
A classic example is the co-pyrolysis of biomass (like wood chips) and plastic waste. Biomass is oxygen-rich but hydrogen-poor, yielding an acidic and unstable bio-oil. Plastics are hydrogen-rich but can be difficult to process alone.
When pyrolyzed together, hydrogen radicals from the decomposing plastic can "donate" themselves to the biomass compounds. This process, known as deoxygenation, produces a higher quality bio-oil with a higher heating value and better stability.
Why Choose Co-Pyrolysis? Key Drivers and Advantages
The decision to implement co-pyrolysis is driven by the desire to optimize outputs and overcome the inherent challenges of processing single waste streams.
Enhancing Product Quality
The most significant advantage is the ability to upgrade the products. By carefully selecting feedstocks, operators can significantly improve the quality of the bio-oil, making it a more viable candidate for biofuel. It can also be used to tailor the properties of bio-char for specific applications, such as soil amendment or filtration.
Improving Process Stability
Some feedstocks, particularly plastics, can melt and form a viscous liquid that causes reactor blockages and operational problems. Blending them with a structured, non-melting feedstock like biomass can create a more stable matrix within the reactor, preventing agglomeration and ensuring smoother operation.
Expanding Feedstock Flexibility
A facility designed for co-pyrolysis is inherently more versatile. It is not dependent on a single, uniform waste stream. This allows it to process a diverse and variable range of inputs, such as agricultural residues, municipal solid waste, and industrial plastics, making the entire operation more economically resilient.
Understanding the Trade-offs and Challenges
While powerful, co-pyrolysis introduces a layer of complexity that requires careful management. It is not a simple "mix and heat" solution.
The Complexity of Feedstock Ratios
The synergistic effects are highly dependent on the mixing ratio of the feedstocks. An incorrect ratio can negate the benefits or even lead to reduced yields. Determining the optimal blend requires significant research, experimentation, and precise process control.
Potential for Unwanted Reactions
While synergy is the goal, blending materials can sometimes produce undesirable byproducts. For example, plastics containing chlorine (like PVC) can introduce corrosive hydrochloric acid into the system and contaminate the final products if not managed properly.
Equipment and Control Requirements
Because the reactions are more complex, co-pyrolysis demands a sophisticated control system. As noted in pyrolysis unit designs, precise PID temperature controllers and sensitive sensors for monitoring gas flow and composition are essential to steer the process toward the desired outcome and ensure consistent results.
Making the Right Choice for Your Goal
Co-pyrolysis is a strategic tool for optimization. The right approach depends entirely on your end objective.
- If your primary focus is improving fuel quality: Co-process a hydrogen-rich material like plastic waste with an oxygen-rich biomass to produce a more stable bio-oil with a higher energy content.
- If your primary focus is waste management flexibility: Design your system to handle variable streams of different organic wastes, allowing you to adapt to changing feedstock availability.
- If your primary focus is producing high-quality bio-char: Experiment with blending different types of biomass or adding specific minerals to create a bio-char with tailored properties for agriculture or filtration.
By treating co-pyrolysis as a method for targeted chemical optimization, you can transform low-value waste streams into valuable, high-performance products.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Thermochemical decomposition of a mixture of organic materials in an oxygen-free environment. |
| Core Benefit | Synergy: Interactions between feedstocks (e.g., hydrogen donation from plastics to biomass) improve outputs. |
| Primary Products | Bio-oil (liquid fuel), Syngas, and Bio-char (solid residue). |
| Key Advantage | Upgrades product quality and offers greater feedstock flexibility compared to single-feedstock pyrolysis. |
| Main Challenge | Requires precise control of feedstock ratios and reaction conditions to achieve desired synergy. |
Ready to optimize your pyrolysis process and transform waste into high-value products? KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Our precise temperature control systems and reactors are designed to help you master the complexities of co-pyrolysis, whether you're working with biomass, plastics, or other mixed feedstocks. Contact our experts today to discuss how our solutions can help you achieve superior product yields and stability.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- How are composites processed using sintering? Engineered Material Solutions Through Advanced Thermal Bonding
- How do tube furnaces or muffle furnaces ensure stoichiometric accuracy during synthesis? Mastering Li4GeO4 & Li4VO4
- What is the temperature of a rotary hearth furnace? Find the Right Heat for Your Process
- At what temperature does wood pyrolysis begin? Control the Process for Biochar, Bio-Oil, or Syngas
- What is a rotary retort furnace? Achieve Superior Uniformity in Continuous Heat Treatment



















