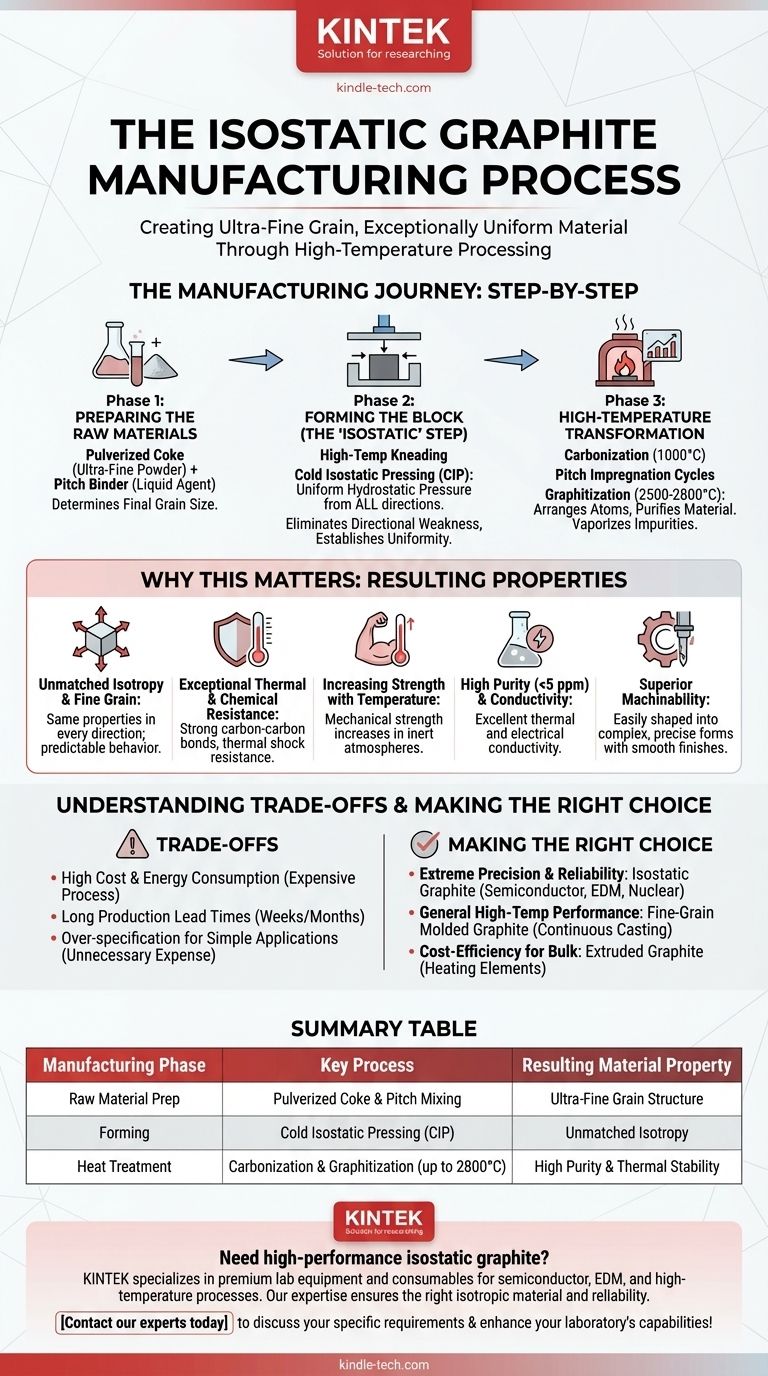
At its core, the manufacturing of isostatic graphite is a multi-stage, high-temperature process designed to create an ultra-fine grain and exceptionally uniform material. It begins by mixing pulverized coke with a pitch binder, which is then compressed under equal pressure from all directions in a Cold Isostatic Press (CIP). This "green" block is then subjected to a series of extreme heat treatments, culminating in a graphitization phase at up to 2800°C, to achieve its final crystalline structure.
The complexity of the isostatic manufacturing process is not a flaw; it is the precise reason for the material's superior, uniform properties. By eliminating the directional grain found in other graphites, this method produces a material with unparalleled isotropy and purity, making it essential for applications where conventional graphites are inadequate.

The Manufacturing Journey, Step-by-Step
The journey from raw powder to a finished, high-purity block is deliberate and controlled. Each phase contributes directly to the final properties of the material.
Phase 1: Preparing the Raw Materials
The foundation of isostatic graphite is a precise mixture of two core components.
First is a high-quality coke, typically from petroleum or coal, which is pulverized into an ultra-fine powder. The final grain size of the graphite is determined at this initial stage.
Second is pitch, a dense, black, liquid by-product of coking coal. This pitch acts as a binder, holding the coke particles together during the subsequent forming process.
Phase 2: Forming the Block (The "Isostatic" Step)
This is the defining stage of the process, where the material's unique uniformity is established.
The pulverized coke and liquid pitch binder are combined in a high-temperature kneading process, creating a homogenous paste-like mixture.
This mixture is then placed in a flexible mold and subjected to Cold Isostatic Pressing (CIP). Unlike extrusion or die-molding, which apply pressure from one or two directions, a CIP applies immense, uniform hydrostatic pressure from all sides. This ensures the coke particles are compacted with no preferential alignment, eliminating inherent directional weakness.
Phase 3: High-Temperature Transformation
The pressed "green" block is not yet graphite. It must undergo a series of intense, energy-consuming heat treatments to transform its structure.
First, the block is carbonized or "baked" at a temperature around 1000°C. This converts the pitch binder into solid carbon, creating a hard, porous carbon block.
To increase density and improve final properties, the block may undergo one or more cycles of pitch impregnation. It is submerged in pitch, which fills the pores, and then re-baked.
Finally, the block is heated in a graphitizing furnace to extreme temperatures, typically 2500°C to 2800°C. This step provides the energy needed to rearrange the amorphous carbon atoms into the ordered, crystalline structure of graphite. This phase also purifies the material, as most impurities vaporize at these temperatures.
Why This Process Matters: The Resulting Properties
The elaborate manufacturing process directly produces a set of unique and highly desirable material characteristics.
Unmatched Isotropy and Fine Grain
The CIP method ensures that the final block has isotropic properties, meaning its mechanical and thermal characteristics (like strength and thermal expansion) are the same in every direction. This, combined with the ultra-fine grain, makes its behavior highly predictable and reliable.
Exceptional Thermal and Chemical Resistance
The strong carbon-carbon bonds formed during graphitization give the material extremely high thermal stability and excellent resistance to chemical attack. It also demonstrates superior thermal shock resistance, withstanding rapid temperature changes without cracking.
Increasing Strength with Temperature
Unlike metals, a key feature of graphite is that its mechanical strength increases with rising temperature in inert atmospheres, making it ideal for high-heat environments like furnaces and rocket nozzles.
High Purity and Conductivity
The high-temperature graphitization process drives off nearly all impurities, allowing for the production of graphite with purity levels less than 5 parts per million (ppm). The well-ordered crystalline structure also ensures high thermal and electrical conductivity.
Superior Machinability
The uniform, fine-grain structure makes isostatic graphite easy to machine into complex and precise shapes with fine detail and smooth surface finishes, which is critical for semiconductor and EDM applications.
Understanding the Trade-offs
While its properties are exceptional, isostatic graphite is not the solution for every problem. Understanding its limitations is key to making an informed decision.
High Cost and Energy Consumption
The multi-stage process, particularly the extremely high temperatures required for graphitization, is very energy-intensive and time-consuming. This makes isostatic graphite significantly more expensive than extruded or vibration-molded graphite.
Long Production Lead Times
The multiple heating, cooling, and impregnation cycles mean that the production process can take several weeks or even months from start to finish. This is a critical factor for project planning and supply chain management.
Over-specification for Simple Applications
For applications that do not require perfect isotropy, extreme purity, or ultra-fine grain, using isostatic graphite can be an unnecessary expense. Simpler, less expensive grades of graphite often provide sufficient performance for applications like casting molds or furnace electrodes.
Making the Right Choice for Your Application
Selecting the correct grade of graphite requires balancing performance requirements with budget and project timelines.
- If your primary focus is extreme precision and reliability: Isostatic graphite is the definitive choice for demanding applications like semiconductor crucibles, nuclear reactor components, or fine-detail Electrical Discharge Machining (EDM).
- If your primary focus is general high-temperature performance: Other fine-grain molded graphites may offer a better balance of cost and performance for applications like continuous casting dies or furnace fixtures.
- If your primary focus is cost-efficiency for bulk components: Extruded graphite, which has directional properties but is much cheaper to produce, is likely the more practical solution for items like heating elements or electrodes.
By understanding the direct link between the manufacturing process and material properties, you can confidently select the precise graphite your project truly demands.
Summary Table:
| Manufacturing Phase | Key Process | Resulting Material Property |
|---|---|---|
| Raw Material Prep | Pulverized Coke & Pitch Mixing | Ultra-Fine Grain Structure |
| Forming | Cold Isostatic Pressing (CIP) | Unmatched Isotropy |
| Heat Treatment | Carbonization & Graphitization (up to 2800°C) | High Purity & Thermal Stability |
Need high-performance isostatic graphite for your precision applications? KINTEK specializes in premium lab equipment and consumables, including high-purity graphite solutions for semiconductor, EDM, and high-temperature processes. Our expertise ensures you get the right material with the isotropic properties and reliability your project demands. Contact our experts today to discuss your specific requirements and discover how KINTEK can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- What is the temperature range of a graphite furnace? Unlock up to 3000°C for advanced materials processing.
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- Can graphite withstand heat? Unlocking its extreme 3,600°C potential in inert environments
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency



















