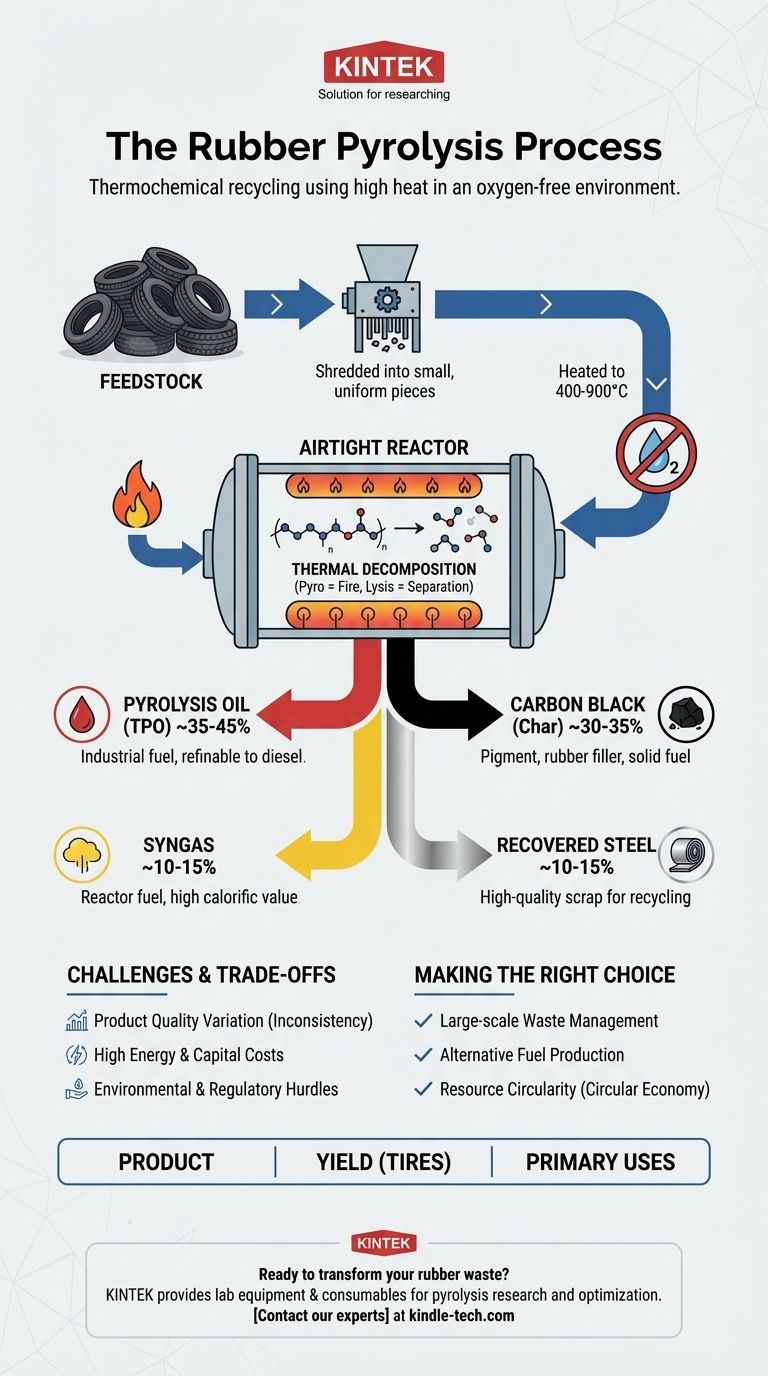
In essence, rubber pyrolysis is a thermochemical recycling process that uses high heat in an oxygen-free environment to decompose waste rubber into valuable raw materials. The process heats shredded rubber, typically from end-of-life tires, to temperatures between 400-900°C, causing the complex polymer chains to break down into simpler, recoverable substances without actually burning the material.
Rubber pyrolysis should not be viewed as simple waste disposal. It is a resource recovery technology that chemically deconstructs rubber waste, primarily tires, transforming a significant environmental liability back into valuable industrial commodities like oil, carbon, and steel.

How Rubber Pyrolysis Works
To understand the process, it's helpful to break it down into its core principles and steps. The name itself—from the Greek words 'pyro' (fire) and 'lysis' (separation)—describes the fundamental action: separating a substance using heat.
The Fundamental Principle: Thermal Decomposition
Think of pyrolysis as "un-baking" the rubber. The vulcanization process that creates a durable tire involves cross-linking long polymer chains with sulfur. Pyrolysis reverses this by applying intense thermal energy.
This energy breaks these long, complex hydrocarbon chains into smaller, simpler molecules. The result is a mixture of gas, liquid hydrocarbons, and solid carbon.
The Critical Role of an Oxygen-Free Environment
The process must occur in a sealed reactor with no oxygen present. This is the key difference between pyrolysis and incineration (burning).
Without oxygen, the rubber cannot combust. Instead of burning and releasing its energy as just heat and smoke, the material decomposes into a new set of stable, valuable chemical products.
A Step-by-Step Overview
- Feedstock Preparation: Waste tires and other rubber products are shredded into small, uniform pieces. This increases the surface area for more efficient and even heating. For tires, the internal steel wiring is often removed at this stage or separated after the process.
- Heating in the Reactor: The shredded rubber is fed into an airtight reactor. The material is then heated to the target temperature (typically 400-900°C), triggering the thermal decomposition.
- Separation and Collection: The resulting substances are separated. Hot vapors are directed through a condensation system to cool and collect the liquid pyrolysis oil. Non-condensable gases (syngas) are routed out, and the solid char and steel remain in the reactor for collection.
What Are the End Products and Their Uses?
The primary value of pyrolysis lies in the outputs it creates. The exact yield depends on the type of rubber and process conditions, but a typical breakdown for tires is a good baseline.
Pyrolysis Oil (~35-45%)
This is a synthetic crude oil, often called Tire Pyrolysis Oil (TPO). It is the main liquid product and can be used directly as an industrial fuel in furnaces or boilers. With further refining, it can be upgraded into more valuable products like diesel.
Carbon Black (~30-35%)
The primary solid residue is a form of crude carbon black, or "char." While not as high-grade as virgin carbon black, it can be used as a pigment, reinforcing filler in lower-spec rubber products, or as a solid fuel (similar to coal).
Syngas (~10-15%)
This mixture of non-condensable, flammable gases (like hydrogen, methane, and carbon monoxide) has a high calorific value. Most modern pyrolysis plants reuse this gas as fuel to power the reactors, significantly reducing the external energy required and making the process more sustainable.
Recovered Steel (~10-15%)
When processing steel-belted tires, the high-quality steel wire is recovered intact. It is a clean, valuable scrap metal that can be easily sold and recycled.
Understanding the Trade-offs and Challenges
While promising, rubber pyrolysis is not a perfect solution and comes with practical challenges that must be considered.
Product Quality and Consistency
The quality of the pyrolysis oil and carbon char can vary significantly based on the input feedstock (e.g., car tires vs. truck tires vs. shoe soles) and minor fluctuations in process temperature and time. This inconsistency can make it difficult to secure off-take agreements with buyers who require a standardized product.
Energy and Capital Costs
Pyrolysis plants require a significant upfront capital investment. Furthermore, the process is energy-intensive, requiring sustained high temperatures. While using the byproduct syngas as fuel helps, the overall energy balance and economic viability must be carefully calculated.
Environmental and Regulatory Hurdles
Although it's a form of recycling, a pyrolysis facility is still a chemical processing plant. It must adhere to strict environmental regulations regarding air emissions and the handling of byproducts. Poorly operated plants can risk creating secondary pollution.
Making the Right Choice for Your Goal
Adopting pyrolysis technology depends entirely on your primary objective.
- If your primary focus is large-scale waste tire management: Pyrolysis is an excellent solution for diverting massive volumes of tires from landfills and converting them into a manageable set of commodities.
- If your primary focus is producing alternative fuels: The process reliably generates a marketable fuel oil, but understand that achieving higher-value fuel grades will require additional investment in refining and purification equipment.
- If your primary focus is resource circularity: This technology is a powerful example of the circular economy, effectively closing the loop by turning a waste product back into hydrocarbons, carbon, and steel.
Ultimately, rubber pyrolysis represents a powerful technical shift from viewing rubber waste as a liability to recognizing it as a valuable and recoverable resource.
Summary Table:
| Product | Typical Yield (from tires) | Primary Uses |
|---|---|---|
| Pyrolysis Oil (TPO) | 35-45% | Industrial fuel for furnaces/boilers; can be refined into diesel |
| Carbon Black (Char) | 30-35% | Filler for rubber products; pigment; solid fuel |
| Syngas | 10-15% | Fuel to power the pyrolysis reactor, reducing external energy needs |
| Recovered Steel | 10-15% | High-quality scrap metal for recycling |
Ready to transform your rubber waste into profit?
KINTEK specializes in providing robust laboratory equipment and consumables to help you research, develop, and optimize pyrolysis processes. Whether you are scaling up from lab testing or need reliable analysis tools, our solutions can help you achieve consistent, high-quality outputs from waste rubber.
Contact our experts today via our Contact Form to discuss how we can support your pyrolysis and resource recovery goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Lab Internal Rubber Mixer Rubber Kneader Machine for Mixing and Kneading
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
People Also Ask
- What are the advantages of blown film extrusion? Boost Your Film Production Efficiency
- How does extrusion work step by step? A Guide to the Continuous Manufacturing Process
- What are the two basic types of extrusion? Hot vs. Cold Extrusion Explained
- What is an internal screw mixer? A Guide to Gentle, Efficient Powder Blending
- What is twin screw granulation? A Guide to Modern, Continuous Pharmaceutical Manufacturing
- What are the advantages of coextrusion? Achieve Multi-Material Efficiency and Superior Performance
- What is the manufacturing process of rubber? From Raw Material to Durable End Product
- Why is the dynamic mixing mode necessary for high-strength HPE-CSPE? Unlock Superior Elastomer Performance



















