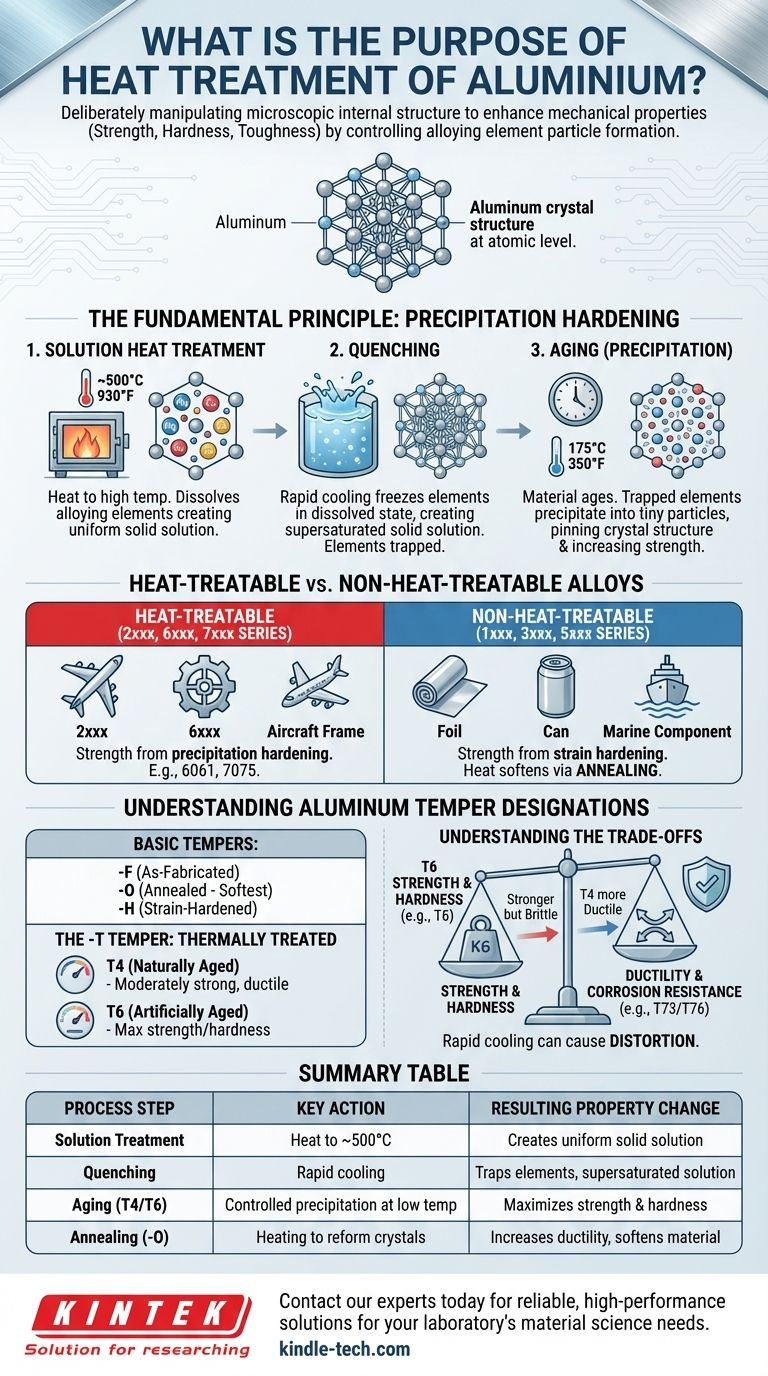
At its core, the purpose of heat treating aluminum is to deliberately manipulate its microscopic internal structure to enhance its mechanical properties. Unlike steel, which is hardened by changing its crystal phase, the most common and effective heat treatments for aluminum alloys increase strength, hardness, and toughness by controlling the formation of tiny particles of alloying elements within the metal.
The central goal of heat treating aluminum is not simply making it harder, but achieving a precise, engineered balance of properties. The process unlocks the high-strength potential of specific aluminum alloys by creating a finely dispersed network of internal precipitates that resist deformation.

The Fundamental Principle: Precipitation Hardening
To understand heat treatment, you must first understand that not all aluminum is the same. The process is only effective on specific "heat-treatable" alloys.
The Problem with Pure Aluminum
Pure aluminum (the 1xxx series) is soft, ductile, and has relatively low strength. While useful for its corrosion resistance and conductivity, it is unsuitable for most structural applications.
Introducing Alloying Elements
To increase its strength, aluminum is mixed with other elements like copper (Cu), magnesium (Mg), and zinc (Zn). In heat-treatable alloys (like the 2xxx, 6xxx, and 7xxx series), these elements can be dissolved into the aluminum and then precipitated out in a controlled manner.
The Three-Step Strengthening Process
This process, known as precipitation hardening or age hardening, is best understood as a three-stage sequence.
-
Solution Heat Treatment: The aluminum alloy is heated to a high, specific temperature (around 500°C / 930°F) and held there. This dissolves the alloying elements into the aluminum, creating a uniform solid solution, much like dissolving sugar in hot water.
-
Quenching: The material is then rapidly cooled, usually in water. This sudden temperature drop freezes the alloying elements in their dissolved state, creating a supersaturated solid solution. The elements are trapped inside the aluminum's crystal lattice, wanting to escape but not having the thermal energy to do so.
-
Aging (Precipitation): In this final, critical step, the material is allowed to "age." The trapped alloying elements begin to clump together and precipitate out of the solution, forming extremely small, numerous, and uniformly dispersed particles. These particles act as obstacles, pinning the crystal structure in place and making it much more difficult for the material to deform. This is what creates the dramatic increase in strength and hardness.
Heat-Treatable vs. Non-Heat-Treatable Alloys
This distinction is critical for any engineering or design decision involving aluminum.
Heat-Treatable Alloys (2xxx, 6xxx, 7xxx Series)
These alloys are designed specifically for precipitation hardening. Their strength is primarily derived from the heat treatment process.
- 2xxx series (Al-Cu): Known for high strength, but generally lower corrosion resistance. Common in aerospace applications.
- 6xxx series (Al-Mg-Si): The workhorse alloys like 6061. They offer a good balance of strength, formability, and corrosion resistance.
- 7xxx series (Al-Zn-Mg): The highest strength aluminum alloys, such as 7075. They are the top choice for high-stress structural components, like aircraft frames.
Non-Heat-Treatable Alloys (1xxx, 3xxx, 5xxx Series)
These alloys gain their strength through strain hardening (work hardening, like rolling or forming) and solid-solution strengthening from their alloying elements. Heat cannot be used to strengthen them.
However, heat can be used to soften these alloys through a process called annealing. This removes the effects of strain hardening, making the material more ductile and easier to form.
Understanding Aluminum Temper Designations
The temper designation, a suffix following the alloy number (e.g., 6061-T6), tells you exactly what has been done to the material.
Basic Tempers: -F, -O, and -H
- -F (As-Fabricated): No special control has been applied to the thermal or strain-hardening conditions.
- -O (Annealed): The softest, most ductile condition, achieved by heating to allow crystals to reform.
- -H (Strain-Hardened): Applies only to non-heat-treatable alloys that have been strengthened by cold working.
The -T Temper: Thermally Treated
The -T designation means the alloy has been heat-treated to produce stable tempers. It is always followed by one or more digits.
- -T4 (Naturally Aged): The material has been solution heat-treated, quenched, and then allowed to age at room temperature. It is reasonably strong but remains ductile enough for some forming operations.
- -T6 (Artificially Aged): After solution treatment and quenching, the material is heated to a low temperature (e.g., 175°C / 350°F) for a specific time. This "artificial aging" accelerates and optimizes the precipitation process, resulting in near-maximum strength and hardness. This is the most common temper for structural aluminum.
Understanding the Trade-offs
Heat treatment is not a free lunch; every enhancement comes with a corresponding trade-off.
Strength vs. Ductility
The primary trade-off is between strength and ductility. A fully aged T6 temper is significantly stronger than a naturally aged T4 temper, but it is also more brittle and cannot be formed as easily. The annealed -O temper is the most ductile but has the lowest strength.
Strength vs. Corrosion Resistance
For some high-strength alloys (especially the 7xxx series), peak strength tempers like T6 can be more susceptible to stress corrosion cracking (SCC). To combat this, special "over-aging" tempers like T73 or T76 are used, which slightly reduce peak strength in exchange for a significant improvement in corrosion resistance.
The Risk of Distortion
The rapid cooling of the quench is a thermal shock that can cause significant distortion and internal stress in complex parts. This requires careful process control, specialized fixtures, and sometimes post-quench straightening or stress-relieving operations.
Making the Right Choice for Your Application
Your choice of alloy and heat treatment must be driven by the end-use requirements of the component.
- If your primary focus is maximum strength and hardness: Choose a heat-treatable alloy like 6061 or 7075 and specify a T6 temper.
- If your primary focus is formability and ductility: Use a non-heat-treatable alloy in an annealed (-O) state, or use a heat-treatable alloy in a T4 temper for forming before aging it to a final temper.
- If your primary focus is balancing strength with stress corrosion resistance: Specify an over-aged temper like T73 for critical 7xxx series components used in corrosive environments.
- If you only need to soften the material for rework or forming: The process you require is annealing, which applies to all aluminum alloys and results in the -O temper.
Understanding these principles empowers you to select the correct material and process to meet your component's specific performance demands.
Summary Table:
| Process Step | Key Action | Resulting Property Change |
|---|---|---|
| Solution Treatment | Heat to ~500°C to dissolve alloying elements | Creates uniform solid solution |
| Quenching | Rapid cooling (e.g., water) | Traps elements, creating supersaturated solution |
| Aging (T4/T6) | Controlled precipitation at low temperature | Maximizes strength and hardness via particle dispersion |
| Annealing (-O) | Heating to reform crystals | Increases ductility and softens material |
Need the right aluminum heat treatment solution for your lab or production? KINTEK specializes in precision lab equipment and consumables for material testing and processing. Our expertise ensures you achieve the exact mechanical properties—whether maximum strength (T6), ductility (-O), or corrosion resistance (T73)—required for your application. Contact our experts today to discuss how we can support your laboratory's material science needs with reliable, high-performance solutions.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What tube is used for tubular furnace? Choose the Right Material for Temperature & Atmosphere
- Why is a horizontal alumina tube furnace ideal for mixed gas corrosion at 650 °C? Ensure Pure Experimental Integrity
- What is the physical description of a tube furnace? A Detailed Breakdown of its High-Temperature Design
- Why use a tube furnace? Achieve Superior Temperature Uniformity and Atmosphere Control
- Why is an Alumina Ceramic Tube Support Necessary for 1100°C Experiments? Ensure Data Accuracy and Chemical Inertness



















