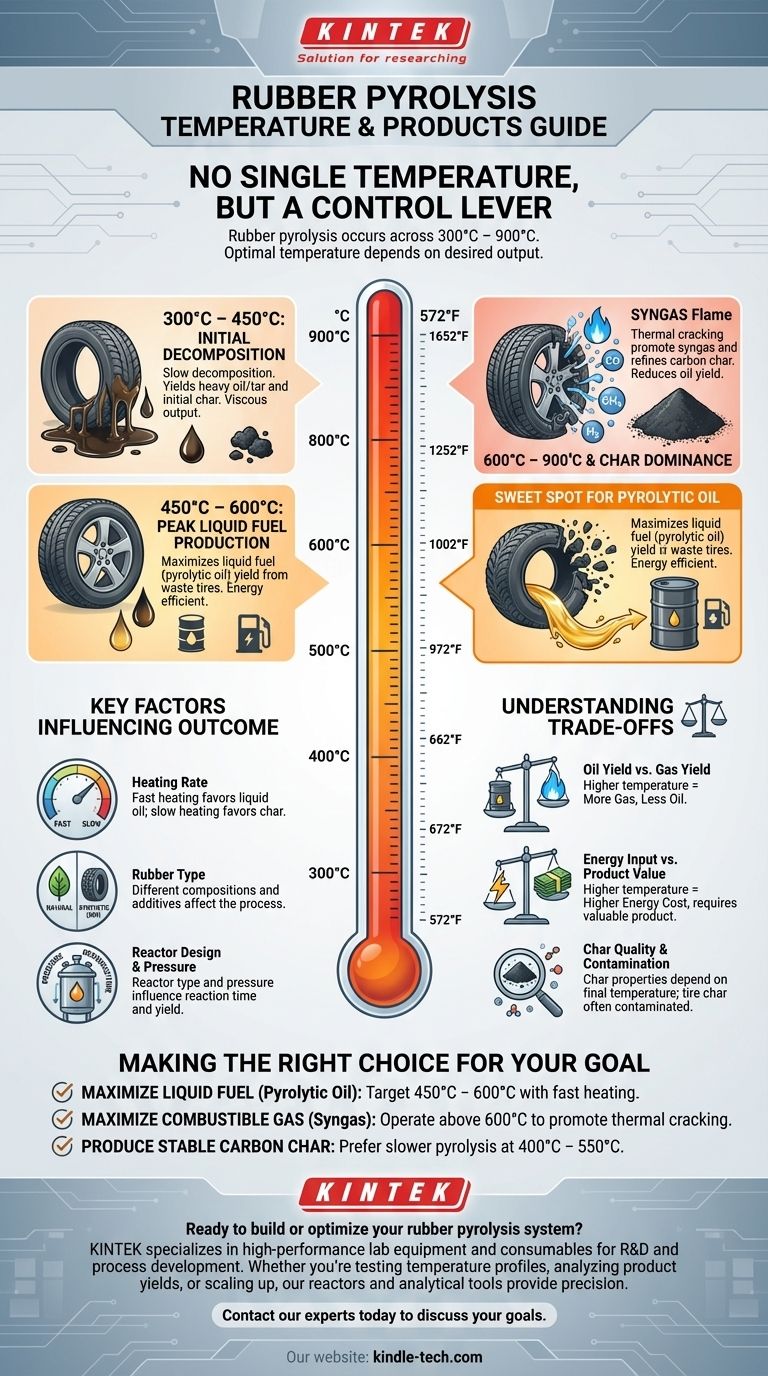
To be precise, there is no single pyrolysis temperature for rubber. Instead, rubber undergoes pyrolysis across a wide temperature range, typically from 300°C to 900°C (572°F to 1652°F). The optimal temperature depends entirely on the desired output, with the most common range for maximizing liquid fuel (pyrolytic oil) being between 400°C and 600°C.
The core principle to understand is that the pyrolysis temperature is not a fixed number but a control lever. Adjusting the temperature changes the primary output, allowing you to selectively produce more liquid oil, combustible gas, or solid carbon char based on your specific goal.

The Stages of Rubber Pyrolysis by Temperature
Pyrolysis is the thermal decomposition of a material in the absence of oxygen. Think of it as carefully taking apart a complex structure with heat, rather than burning it. For rubber, particularly scrap tires, this process breaks down long polymer chains into valuable smaller components.
Initial Decomposition (300°C – 450°C)
At this lower end of the spectrum, the rubber begins to soften and decompose. The weaker chemical bonds break first, releasing heavy, long-chain volatile compounds.
The process is relatively slow, and the output is often a more viscous, tar-like liquid. Char production begins to form as the basic carbon structure is left behind.
Peak Liquid Fuel Production (450°C – 600°C)
This range is often considered the "sweet spot" for producing pyrolytic oil, a synthetic crude oil. The thermal energy is sufficient to efficiently break down the complex hydrocarbons of the rubber into smaller, more valuable liquid molecules.
Commercial operations targeting liquid fuel from waste tires typically operate within this window to maximize their primary product yield and energy efficiency.
Gas and Char Dominance (600°C – 900°C)
As temperatures exceed 600°C, a secondary reaction called thermal cracking becomes dominant. The liquid hydrocarbons produced at lower temperatures are further broken down into very simple, light, non-condensable gases.
This significantly increases the yield of syngas (a mix of hydrogen, carbon monoxide, methane, etc.) but comes at the direct expense of the liquid oil yield. The properties of the solid carbon char residue are also refined at these higher temperatures.
Key Factors That Influence the Outcome
Temperature is the primary variable, but it does not act alone. Several other factors critically influence the efficiency and final product distribution of the pyrolysis process.
Heating Rate
The speed at which the rubber is heated is crucial. Fast pyrolysis (high heating rate) typically favors the production of liquid oil, as it quickly vaporizes the compounds before they can undergo secondary reactions. Slow pyrolysis (low heating rate) tends to produce more solid carbon char.
Type of Rubber
Different types of rubber have different chemical compositions. A natural rubber will behave differently from a synthetic one like SBR (styrene-butadiene rubber), which is a primary component in car tires. The presence of additives, fillers, and steel wires in tires also affects the process and the purity of the final products.
Reactor Design and Pressure
The type of reactor used (e.g., batch, rotary kiln, screw) and the operating pressure can influence how long the vapors remain in the hot zone, which in turn affects the extent of secondary cracking and the final product yields.
Understanding the Trade-offs
Choosing a pyrolysis temperature is an engineering decision based on balancing costs, goals, and outcomes.
Oil Yield vs. Gas Yield
This is the most direct trade-off. Pushing the temperature higher to get more combustible gas will inevitably reduce your liquid fuel output. The economic value of the gas must be weighed against the value of the lost oil.
Energy Input vs. Product Value
Reaching and maintaining higher temperatures requires a significant amount of energy. The operational cost of running a reactor at 800°C is much higher than at 500°C. This extra energy cost must be justified by the value of the products (e.g., high-quality syngas or specialized carbon char).
Char Quality and Contamination
The solid residue, a form of carbon black or char, has potential value as a fuel, a filler, or even activated carbon. Its properties, such as surface area and purity, are highly dependent on the final temperature. However, the char from tires is often contaminated with the silica, zinc, and sulfur used in tire manufacturing.
Making the Right Choice for Your Goal
Ultimately, the ideal temperature is determined by your primary objective.
- If your primary focus is maximizing liquid fuel (pyrolytic oil): Target a temperature range of 450°C to 600°C combined with a relatively fast heating rate.
- If your primary focus is maximizing combustible gas (syngas): Operate at higher temperatures, typically above 600°C, to promote the secondary cracking of oil vapors.
- If your primary focus is producing a stable carbon char: A slower pyrolysis process at lower to moderate temperatures (400°C to 550°C) is often preferred to preserve the carbon structure.
By understanding these principles, you can transform rubber waste into a predictable and valuable resource.
Summary Table:
| Temperature Range | Primary Product | Key Characteristics |
|---|---|---|
| 300°C – 450°C | Heavy Oil / Tar | Slow decomposition, viscous liquid, initial char formation |
| 450°C – 600°C | Pyrolytic Oil | Maximizes liquid fuel yield, efficient for waste tires |
| 600°C – 900°C | Syngas / Char | Promotes gas production via thermal cracking, refines char properties |
Ready to build or optimize your rubber pyrolysis system? KINTEK specializes in high-performance lab equipment and consumables for R&D and process development. Whether you're testing temperature profiles, analyzing product yields, or scaling up from the lab, our reactors, furnaces, and analytical tools provide the precision and reliability you need.
Contact our experts today to discuss how we can support your specific laboratory and pyrolysis application goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time



















