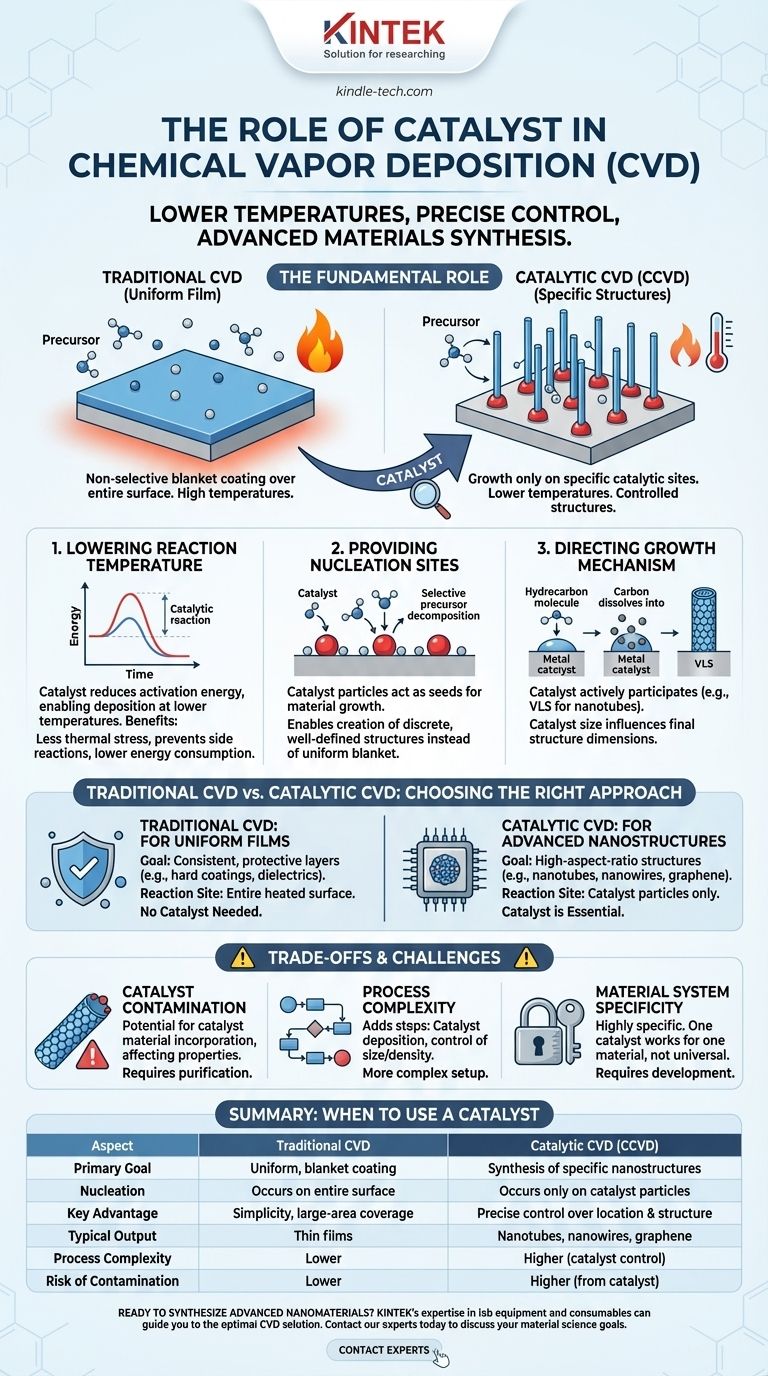
In short, the primary role of a catalyst in Chemical Vapor Deposition (CVD) is to lower the reaction temperature and precisely control the growth of specific material structures. By providing an energetically favorable site for precursor molecules to decompose, a catalyst enables the synthesis of advanced materials, such as carbon nanotubes and nanowires, that are not possible with traditional CVD.
A catalyst transforms CVD from a simple surface-coating technique into a sophisticated material synthesis process. It dictates where growth begins, how it proceeds, and often determines the final structure of the deposited material itself.

The Fundamental Role of a Catalyst in CVD
While standard CVD creates uniform films over an entire surface, catalytic CVD (CCVD) uses a catalyst to achieve highly specific outcomes. This is accomplished through several key mechanisms.
Lowering the Reaction Temperature
A catalyst significantly reduces the activation energy required to break down the precursor gas. This means the deposition can occur at much lower temperatures than in conventional thermal CVD.
This is a critical advantage, as lower temperatures reduce thermal stress on the substrate, prevent unwanted side reactions, and dramatically lower energy consumption.
Providing Nucleation and Growth Sites
In catalytic CVD, the catalyst particles (often metal nanoparticles) act as "seeds" for material growth. The precursor gas selectively decomposes on the surface of these catalyst particles, not on the surrounding substrate.
This provides a powerful mechanism for control. Instead of a uniform blanket coating, the material grows only from these specific catalytic sites, enabling the creation of discrete, well-defined structures.
Directing the Growth Mechanism
The catalyst is not just a passive site; it actively participates in the growth process. A classic example is the growth of carbon nanotubes.
A hydrocarbon precursor (like acetylene) breaks down on a metal nanoparticle (like iron). The carbon atoms dissolve into the metal particle until it becomes supersaturated. The carbon then precipitates out from the particle, forming the cylindrical wall of a nanotube. The size of the catalyst particle directly influences the diameter of the resulting nanotube.
Traditional CVD vs. Catalytic CVD
Understanding when to use a catalyst requires distinguishing between the goals of uniform coating and controlled synthesis.
Traditional CVD: For Uniform Films
When the objective is to apply a consistent, uniform layer over an entire component—such as a hard, protective coating or a dielectric film—traditional CVD is used.
In this case, the entire heated substrate surface acts as the reaction site. No specific catalyst is needed because the goal is non-selective, blanket deposition.
Catalytic CVD: For Advanced Nanostructures
When the goal is to synthesize specific, high-aspect-ratio structures like nanowires, nanotubes, or high-quality graphene sheets, a catalyst is essential.
Here, the catalyst’s ability to control nucleation location and growth direction is the key to forming these complex, bottom-up structures.
Understanding the Trade-offs and Challenges
While powerful, using a catalyst introduces complexities and potential downsides that must be managed.
Catalyst Contamination
The most significant drawback is the potential for the catalyst material to be incorporated into the final product as an impurity.
For example, residual metal catalyst at the base or tip of a carbon nanotube can negatively affect its electronic or mechanical properties. This often requires additional post-processing steps to purify the material.
Process Complexity
Introducing a catalyst adds steps and variables to the CVD process. The catalyst material must first be deposited onto the substrate (e.g., via sputtering or evaporation) and its size, density, and distribution must be carefully controlled.
This adds a layer of complexity compared to the more straightforward setup of traditional CVD.
Material System Specificity
Catalysis in CVD is a highly specific chemical process. A particular catalyst-precursor combination is typically optimized for growing only one type of material.
A catalyst that works for growing silicon nanowires will not work for growing carbon nanotubes. This lack of universality means that significant process development is required for each new material system.
Making the Right Choice for Your Goal
The decision to use a catalyst is determined entirely by the material you intend to create.
- If your primary focus is a uniform, protective film over a large area: Traditional thermal or plasma-enhanced CVD is the appropriate choice, as a catalyst is unnecessary and only adds complexity.
- If your primary focus is synthesizing specific nanostructures like nanotubes or nanowires: Catalytic CVD is not just an option, but a fundamental requirement for controlling the location, size, and mechanism of growth.
Ultimately, understanding the role of the catalyst empowers you to select the correct deposition strategy to achieve your specific material science objective.
Summary Table:
| Aspect | Traditional CVD | Catalytic CVD (CCVD) |
|---|---|---|
| Primary Goal | Uniform, blanket coating | Synthesis of specific nanostructures |
| Nucleation | Occurs on entire substrate surface | Occurs only on catalyst particles |
| Key Advantage | Simplicity, large-area coverage | Precise control over growth location & structure |
| Typical Output | Thin films | Nanotubes, nanowires, graphene |
| Process Complexity | Lower | Higher (requires catalyst deposition & control) |
| Risk of Contamination | Lower | Higher (from catalyst material) |
Ready to Synthesize Advanced Nanomaterials?
Choosing the right CVD process is critical for your research and development. Whether you need to create uniform protective coatings or synthesize complex nanostructures like carbon nanotubes, KINTEK's expertise in lab equipment and consumables can guide you to the optimal solution.
Contact our experts today to discuss your specific material science goals and discover how our specialized CVD systems and support can accelerate your innovation.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings



















