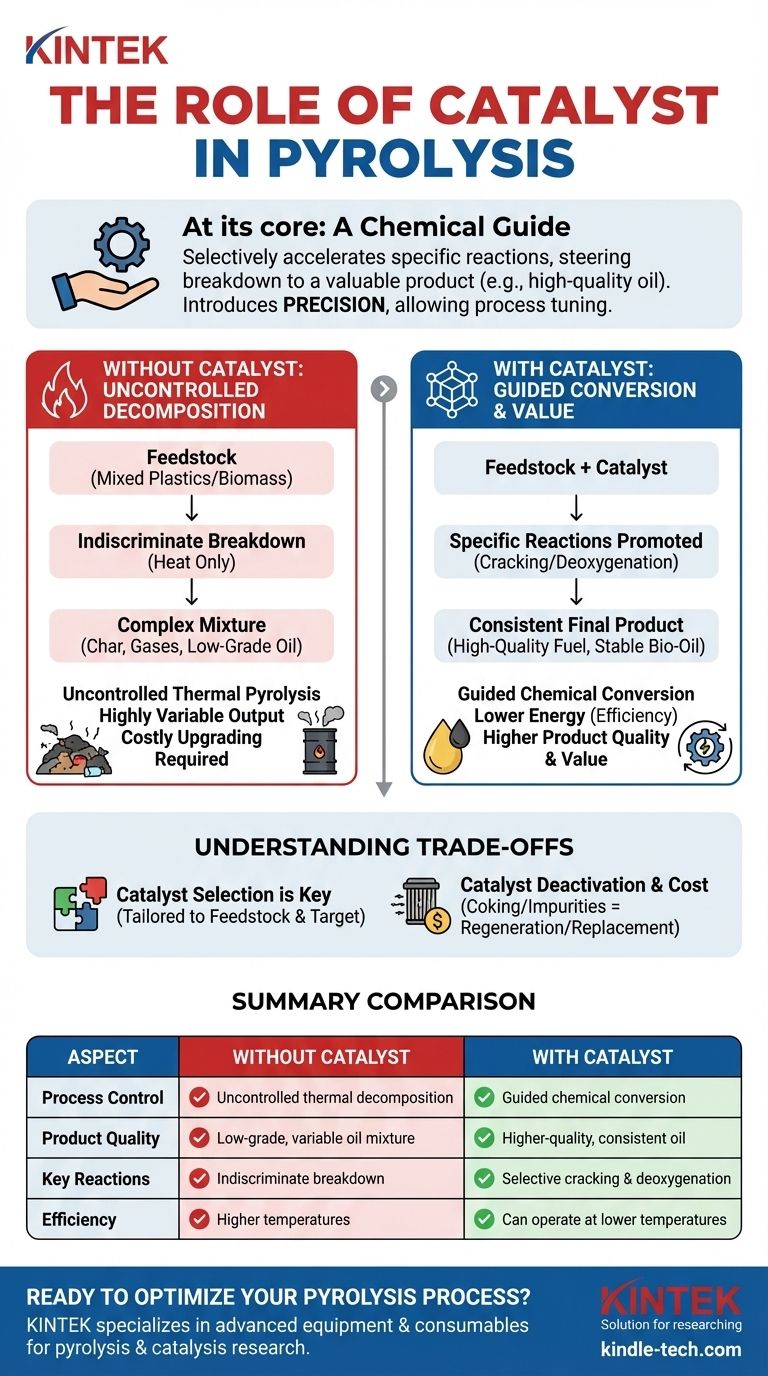
At its core, the role of a catalyst in pyrolysis is to act as a chemical guide. It selectively accelerates specific reactions, steering the breakdown of raw materials like plastic or biomass toward a more valuable and consistent final product, such as high-quality oil.
Pyrolysis without a catalyst is an uncontrolled thermal decomposition, often resulting in a low-quality, highly variable output. A catalyst introduces precision, allowing operators to "tune" the process and target the production of specific, desirable chemical compounds.

The Challenge of Uncontrolled Pyrolysis
To understand why catalysts are so critical, we must first look at the nature of pyrolysis when left to its own devices.
What Happens Without a Catalyst
In simple thermal pyrolysis, heat is the only tool used to break down the feedstock. This process is effective at decomposition, but it is not precise.
The result is often a complex mixture of many different compounds, including undesirable char, gases, and a low-grade oil that may require significant and costly upgrading before it can be used.
The Problem of Feedstock Variability
Materials like mixed plastics or biomass are not uniform. Their chemical compositions vary widely, and thermal pyrolysis breaks them down indiscriminately.
This variability in the input leads directly to an inconsistent and unpredictable output, making it difficult to create a reliable and valuable product stream.
How Catalysts Provide Control and Value
Introducing a catalyst fundamentally changes the process from simple decomposition to a guided chemical conversion.
Promoting Specific Chemical Reactions
The primary function of a catalyst is to provide a surface or pathway that lowers the energy required for a specific type of chemical reaction to occur.
In the context of converting plastic to oil, catalysts promote cracking reactions. This breaks down long, heavy hydrocarbon chains into smaller, more valuable ones that are characteristic of gasoline or diesel fuels.
Improving Final Product Quality
For biomass, a major issue is the high oxygen content in the resulting bio-oil, which makes it acidic and unstable. Catalysts can promote deoxygenation reactions, removing oxygen and creating a more stable, energy-dense product similar to crude oil.
This selective action produces a higher-quality oil with a more desirable and narrow range of compounds, reducing the need for extensive downstream processing.
Increasing Process Efficiency
By lowering the activation energy for desired reactions, catalysts can often allow the pyrolysis process to run at lower temperatures.
This can lead to significant energy savings, reducing operational costs and improving the overall economic viability of the conversion process.
Understanding the Trade-offs
While catalysts are powerful tools, they are not a universal solution. Their application requires careful consideration.
Catalyst Selection is Key
There is no single "best" catalyst for all pyrolysis applications. The choice of catalyst must be tailored to the specific feedstock and the desired final product.
The variability of materials like biomass or mixed plastics necessitates tunable catalysts that can be optimized to favor the reactions needed to produce a specific target compound. An improper choice can lead to unwanted byproducts or inefficiency.
Catalyst Deactivation and Cost
Catalysts have a finite lifespan. Over time, their active sites can become blocked by carbon deposits (a process called coking) or contaminated by impurities in the feedstock.
This deactivation means catalysts must be periodically regenerated or replaced, adding complexity and cost to the operation. The initial cost of the catalyst itself is also a significant economic factor.
How to Apply This to Your Goal
Your choice to use a catalyst—and which one you select—depends entirely on your primary objective.
- If your primary focus is converting mixed plastics into a specific fuel range: You need a catalyst, like a zeolite, designed for cracking long hydrocarbon chains into shorter, more uniform molecules.
- If your primary focus is upgrading bio-oil from biomass: Your goal is to remove oxygen, so you should select a catalyst that promotes deoxygenation and stabilization reactions.
- If your primary focus is simply volume reduction with minimal processing: Non-catalytic thermal pyrolysis may be sufficient, but you must accept a lower-quality and less valuable liquid product.
Ultimately, incorporating a catalyst transforms pyrolysis from a brute-force decomposition method into a refined and controllable chemical manufacturing process.
Summary Table:
| Aspect | Without Catalyst | With Catalyst |
|---|---|---|
| Process Control | Uncontrolled thermal decomposition | Guided chemical conversion |
| Product Quality | Low-grade, variable oil mixture | Higher-quality, consistent oil |
| Key Reactions | Indiscriminate breakdown | Selective cracking & deoxygenation |
| Efficiency | Higher temperatures often required | Can operate at lower temperatures |
Ready to optimize your pyrolysis process for maximum value?
At KINTEK, we specialize in providing advanced laboratory equipment and consumables tailored to your specific pyrolysis and catalysis research needs. Whether you are converting plastics into fuel or upgrading biomass into stable bio-oil, our solutions help you achieve precise control and superior product quality.
Contact our experts today to discuss how we can support your goals with the right tools and expertise.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Custom PTFE Teflon Parts Manufacturer for Culture Dish and Evaporation Dish
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















