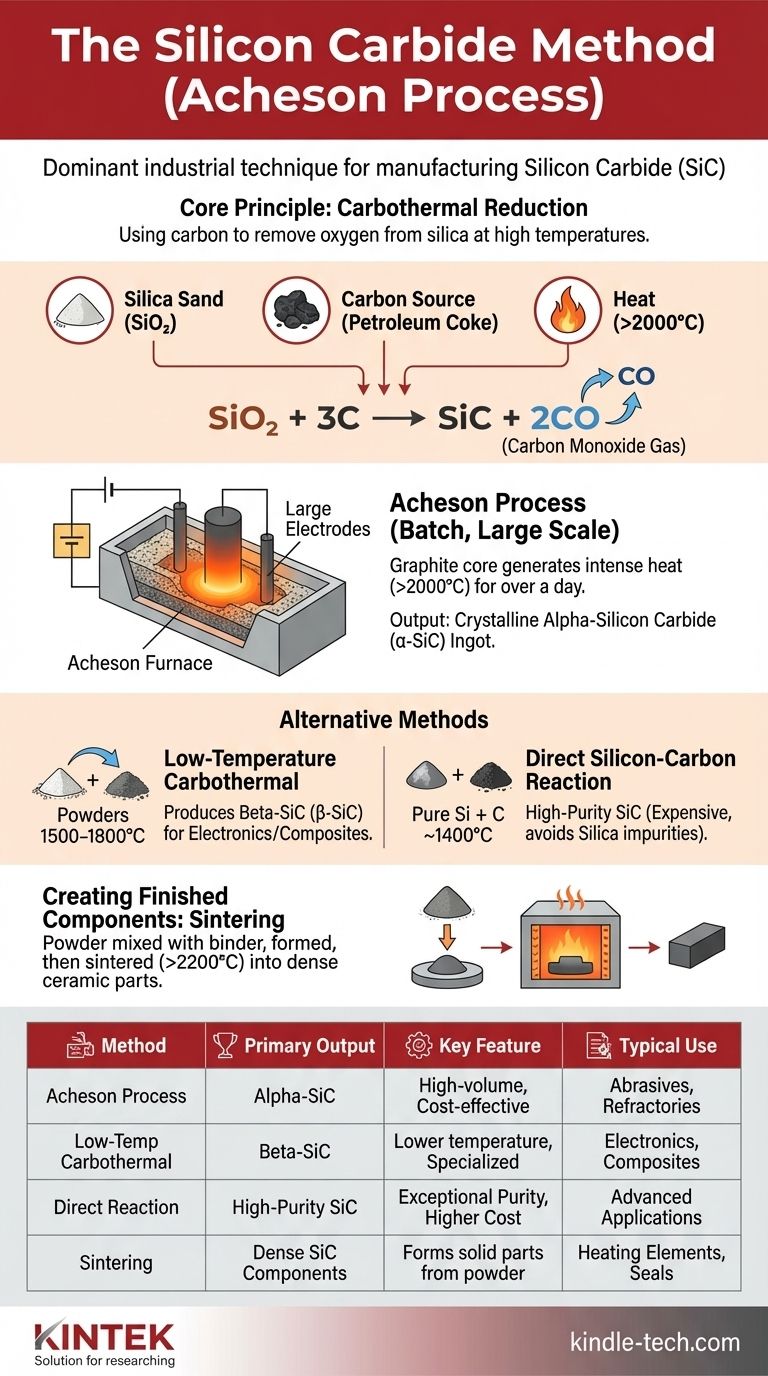
In short, the "silicon carbide method" most commonly refers to the Acheson process, the dominant industrial technique for manufacturing silicon carbide (SiC). This method involves the high-temperature reaction of silica sand and carbon in a large electric resistance furnace. While other specialized methods exist, the Acheson process is the foundational technology for producing the vast majority of SiC used in industry today.
Silicon carbide is a synthetic material, meaning it must be manufactured. All production methods are based on a fundamental chemical principle: using a carbon source to remove oxygen from silica at extremely high temperatures, which allows the remaining silicon and carbon to bond into a new, exceptionally hard compound.

The Core Principle: Carbothermal Reduction
The Key Ingredients
The raw materials for silicon carbide production are simple and abundant. The primary inputs are high-purity silica (silicon dioxide, SiO₂) sourced from quartz sand and a source of carbon, typically petroleum coke.
The Fundamental Reaction
At its heart, the process is a carbothermal reduction. In the intense heat of a furnace, the carbon reacts with the silica, "stealing" the oxygen atoms to form carbon monoxide (CO) gas.
This frees the silicon to bond directly with the excess carbon, creating silicon carbide. The simplified chemical reaction is: SiO₂ + 3C → SiC + 2CO.
Primary Industrial Production: The Acheson Method
The Acheson method, developed in the 1890s, remains the workhorse for bulk SiC production. It is a batch process defined by its unique furnace design and massive scale.
The Furnace Setup
A typical Acheson furnace is a large, trough-like structure, often over 40 feet long. It is loaded with a precise mixture of silica sand and petroleum coke. A central core of graphite is buried within this mixture, running from end to end.
The Heating Process
An enormous electric current is passed through the graphite core. The core acts as a resistor, generating immense heat and raising the internal temperature of the mixture to over 2000°C (3600°F).
This extreme temperature initiates the carbothermal reduction, which proceeds for over a day. The reaction consumes the raw materials, forming a large, crystalline ingot of silicon carbide around the central core.
The Outcome: Alpha-Silicon Carbide (α-SiC)
After cooling, the furnace is disassembled. The result is a hollow cylinder of intergrown silicon carbide crystals. This raw ingot is then mechanically crushed, cleaned, and sorted by size for various applications.
The Acheson process primarily produces alpha-silicon carbide (α-SiC), the most common and thermodynamically stable crystalline form of the material, known for its extreme hardness.
Alternative Synthesis Methods
While the Acheson process dominates, other methods are used to produce different grades or forms of SiC for specialized applications.
Low-Temperature Carbothermal Reduction
This method reacts fine silica and carbon powders at lower temperatures, typically between 1500°C and 1800°C. It is used to synthesize beta-silicon carbide (β-SiC), a different crystal structure often preferred for certain electronic or composite applications.
Direct Silicon-Carbon Reaction
For applications demanding exceptional purity, SiC can be made by reacting pure metallic silicon powder directly with carbon powder at temperatures around 1400°C. This avoids using silica sand, eliminating a source of impurities, but is significantly more expensive due to the cost of pure silicon.
Creating Finished Components
The methods above produce SiC powder. To create solid parts like heating rods or mechanical seals, this powder is mixed with a binder, formed into the desired shape, and then sintered. Sintering is a high-temperature process (up to 2200°C) that causes the individual SiC grains to bond and recrystallize, forming a dense, solid ceramic component.
Understanding the Trade-offs
Purity vs. Cost
The Acheson method is the most cost-effective for large volumes, making it ideal for industrial abrasives and refractories. However, its purity is limited by the raw materials. Direct reaction methods produce higher purity SiC but at a much higher cost.
Crystal Structure (α-SiC vs. β-SiC)
Alpha-SiC, produced by the Acheson method, is the harder, more stable polymorph used for most structural and abrasive roles. Beta-SiC is a cubic crystal form that is valuable in producing fine powders and has specific uses in advanced composites and semiconductor research.
Energy Consumption
All silicon carbide synthesis methods are extremely energy-intensive. The need to achieve and maintain temperatures well above 1500°C makes energy a primary cost driver and a significant environmental consideration in SiC production.
Making the Right Choice for Your Goal
Understanding the production method is key to selecting the correct material for your application.
- If your primary focus is industrial abrasives, sandblasting media, or refractory bricks: The cost-effective α-SiC produced via the Acheson method is the industry standard.
- If your primary focus is high-purity material for advanced electronics or composites: The more expensive β-SiC from direct reaction or specialized carbothermal methods is the appropriate choice.
- If your primary focus is a finished high-temperature component like a heating element: The critical process is the sintering of SiC powder, which occurs after the initial synthesis and determines the final density and strength.
Ultimately, knowing how silicon carbide is made allows you to understand the inherent properties, purity, and cost structure of the material you are working with.
Summary Table:
| Method | Primary Output | Key Feature | Typical Use |
|---|---|---|---|
| Acheson Process | Alpha-SiC (α-SiC) | High-volume, cost-effective | Abrasives, refractories |
| Low-Temperature Carbothermal | Beta-SiC (β-SiC) | Lower temperature, specialized | Electronics, composites |
| Direct Reaction | High-Purity SiC | Exceptional purity, higher cost | Advanced applications |
| Sintering | Dense SiC Components | Forms solid parts from powder | Heating elements, seals |
Need high-quality silicon carbide materials or expert advice for your lab? KINTEK specializes in lab equipment and consumables, providing the right SiC products for applications ranging from industrial abrasives to high-purity electronic components. Let our expertise help you select the optimal material for your specific needs—contact us today to discuss your requirements!
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the factors influencing shrinkage during sintering? Control Dimensional Changes for Precision Parts
- Why is a high vacuum required for sintering Ti-43Al-4Nb-1Mo-0.1B? Ensure Purity & Fracture Toughness
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- Why is a high vacuum environment necessary in sintering equipment for TiAl alloys? Ensure High-Purity Metal Bonding
- How does a high-temperature vacuum sintering furnace facilitate the post-treatment of Zirconia coatings?



















