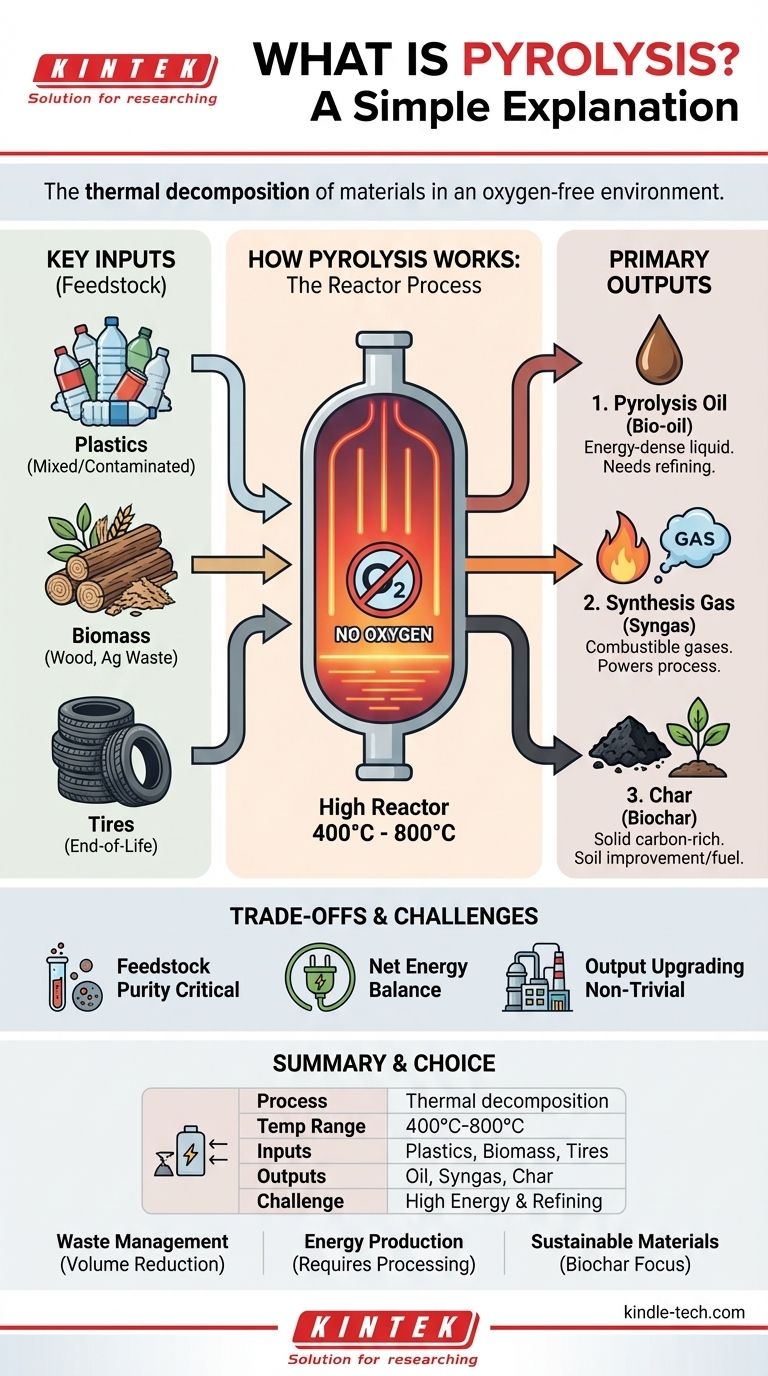
In essence, pyrolysis is the thermal decomposition of materials at high temperatures in an oxygen-free environment. Think of it not as burning, but as a controlled "baking" process that breaks down complex materials like plastic or biomass into simpler, more valuable substances. The absence of oxygen is the critical factor that prevents combustion and instead forces a chemical transformation.
Pyrolysis is fundamentally a conversion technology. Its primary function is to transform low-value or problematic materials, such as plastic waste or agricultural residues, into a portfolio of useful energy products and chemical feedstocks.

How Pyrolysis Works: A Look Inside the Reactor
To truly grasp pyrolysis, we must look at it as a controlled chemical engineering process. It takes a specific input, subjects it to precise conditions, and yields a predictable set of outputs.
The Principle of Thermal Decomposition
The process begins by feeding material, known as feedstock, into a reactor. This vessel is then sealed to remove oxygen.
High heat (typically between 400°C and 800°C) is applied. This intense thermal energy, without oxygen to facilitate burning, breaks the strong chemical bonds within the large molecules of the feedstock.
This molecular breakdown, often compared to the thermal cracking used in petroleum refining, rearranges the material into smaller, less complex molecules.
The Key Inputs (Feedstock)
The versatility of pyrolysis lies in its ability to process various organic materials. Common feedstocks include:
- Plastics: Especially mixed or contaminated plastics that are difficult to recycle mechanically.
- Biomass: Wood, agricultural waste (corn stover, rice husks), and other plant-based matter.
- Tires: End-of-life tires are a common feedstock due to their high energy content.
The Three Primary Outputs
The process does not destroy the material but rather separates it into three distinct product streams.
1. Pyrolysis Oil (Bio-oil): A dark, viscous liquid that is chemically similar to a crude fossil oil. It is energy-dense but requires further refining before it can be used as a transportation fuel.
2. Synthesis Gas (Syngas): A mixture of non-condensable, combustible gases, primarily hydrogen, carbon monoxide, and methane. This gas can be burned on-site to provide the heat needed to power the pyrolysis process itself, improving its energy efficiency.
3. Char (Biochar): A solid, carbon-rich material resembling charcoal. When derived from biomass, this "biochar" can be used to improve soil quality and sequester carbon. When derived from plastics or tires, it is typically used as a solid fuel.
Understanding the Trade-offs and Challenges
While promising, pyrolysis is not a silver bullet. An objective assessment requires understanding its operational complexities and limitations.
Feedstock Purity is Critical
Pyrolysis reactors are sensitive to the composition of their feedstock. Contaminants, such as certain types of plastics (like PVC) or metals, can produce corrosive acids and toxic compounds, damaging equipment and creating hazardous byproducts.
The Net Energy Balance
The process is energy-intensive, requiring a significant thermal input to maintain its high operating temperatures. A successful pyrolysis operation must be able_ to generate more energy from its outputs than it consumes, a concept known as a positive net energy balance.
Output Upgrading is Non-Trivial
The liquid pyrolysis oil is not a direct, "drop-in" replacement for diesel or gasoline. It is often acidic, unstable, and contains oxygenates and water, which must be removed through a costly and complex secondary process called upgrading or hydrotreating.
Making the Right Choice for Your Goal
Evaluating pyrolysis depends entirely on your specific objective. The technology's value proposition changes based on whether you are focused on waste, energy, or materials.
- If your primary focus is waste management: View pyrolysis as a powerful tool for volume reduction and for converting non-recyclable materials into stable, more manageable outputs.
- If your primary focus is energy production: Recognize that pyrolysis generates multiple energy streams, but the liquid fuel requires significant post-processing to become a viable commercial product.
- If your primary focus is sustainable materials: Focus on the solid biochar output from biomass, which has growing applications in carbon sequestration, soil amendment, and advanced manufacturing.
By understanding pyrolysis as a precise chemical conversion process rather than simple disposal, you can accurately evaluate its role in a sustainable, circular economy.
Summary Table:
| Aspect | Description |
|---|---|
| Process | Thermal decomposition of materials in an oxygen-free environment. |
| Temperature Range | Typically 400°C to 800°C. |
| Key Inputs (Feedstock) | Plastics, biomass (wood, agricultural waste), tires. |
| Primary Outputs | Pyrolysis Oil (Bio-oil), Synthesis Gas (Syngas), Char (Biochar). |
| Main Challenge | Requires high energy input and often needs further refining of outputs. |
Ready to explore pyrolysis solutions for your laboratory or pilot project?
KINTEK specializes in providing high-quality lab equipment and consumables for advanced thermal processes like pyrolysis. Whether you are researching waste conversion, developing new biofuels, or optimizing material outputs, our reactors and analytical tools are designed for precision, safety, and reliability.
We can help you achieve accurate and reproducible results in your pyrolysis experiments. Contact our experts today to discuss your specific application and how our equipment can drive your research forward.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What temperature is tube annealing? A Guide to Material-Specific Ranges for Optimal Results
- How do you clean a tubular furnace tube? A Step-by-Step Guide to Safe and Effective Maintenance
- How do you clean a quartz tube furnace? Prevent Contamination & Extend Tube Lifespan
- What is the difference between upflow and horizontal furnace? Find the Perfect Fit for Your Home's Layout
- What is quartz tube heating? Achieve Instant, Targeted Heat with Infrared Radiation



















