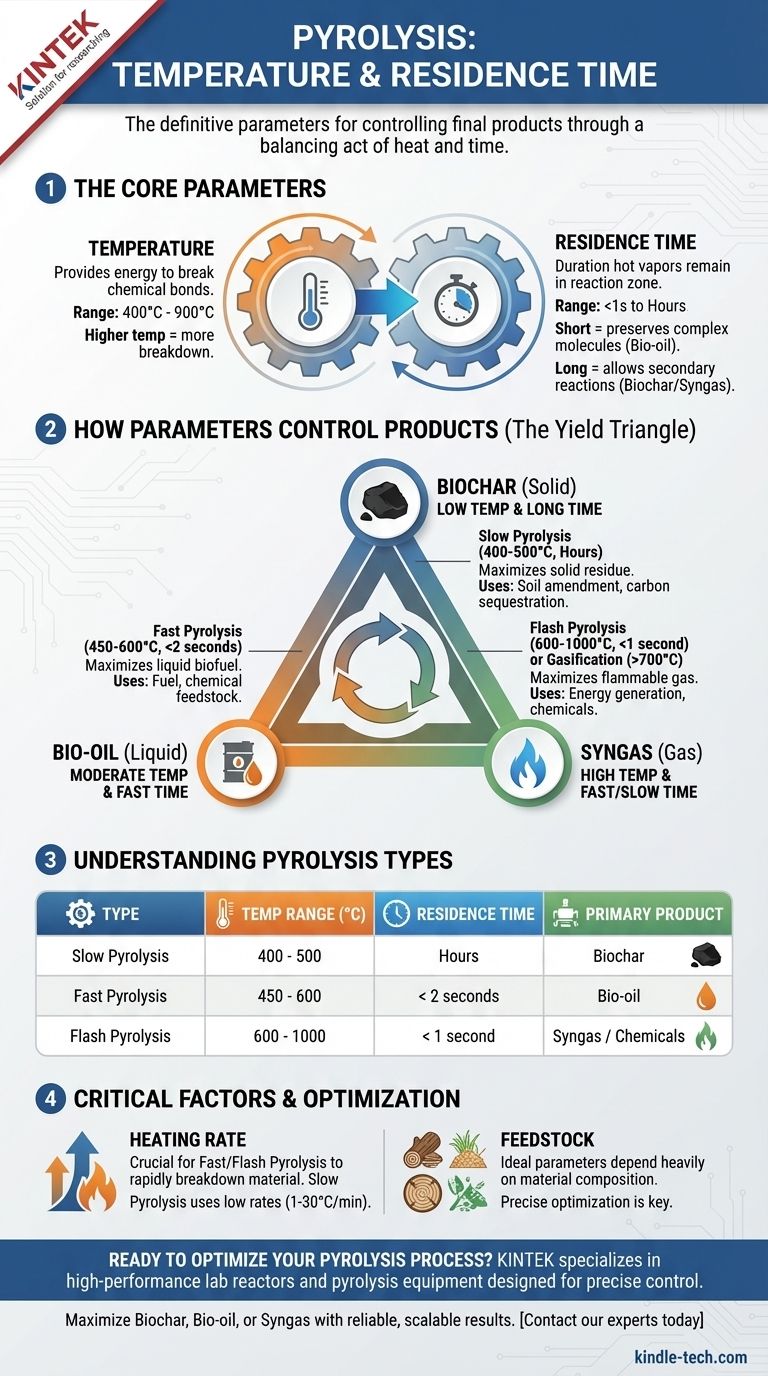
The definitive parameters for pyrolysis are a temperature between 400-900°C and a vapor residence time ranging from less than a second to several hours. These two variables are not independent; they are intentionally manipulated in a trade-off to control the final products. The specific temperature and time you choose are determined entirely by whether you want to maximize the output of biochar, bio-oil, or syngas.
The core principle of pyrolysis is a balancing act between heat and time. Low temperatures and long residence times produce solid biochar, while high temperatures and short residence times produce liquid bio-oil. Pushing the temperature even higher favors the production of gas.
The Core Parameters: Temperature and Time
Pyrolysis is the thermal decomposition of materials in an oxygen-free environment. Understanding how temperature and time interact is the key to controlling the outcome.
Temperature's Role in Decomposition
Temperature provides the necessary energy to break the chemical bonds within the feedstock. While initial breakdown for materials like wood can begin as low as 200-300°C, effective and complete pyrolysis typically requires a higher thermal range.
The process is generally conducted between 400°C and 900°C. Lower temperatures are insufficient for rapid conversion, while temperatures above this range often move into the realm of gasification, where the primary goal is to create syngas.
Residence Time's Role in Product Formation
Residence time refers to how long the hot pyrolysis vapors remain in the reaction zone before being cooled and collected. This variable is arguably as important as temperature.
A short residence time (seconds or less) is crucial for preserving the complex molecules that form liquid bio-oil. The vapors are removed and quenched quickly before they can break down further into simpler, non-condensable gases.
A long residence time (minutes to hours) allows for secondary reactions to occur. The initial vapors crack and re-polymerize, forming more biochar and simple gases, thereby reducing the final liquid yield.
How Different Pyrolysis Types Control Products
The specific combination of temperature and residence time defines the type of pyrolysis and its primary output.
Slow Pyrolysis: Maximizing Biochar
Slow pyrolysis uses lower temperatures (around 400-500°C) and very long residence times (from many minutes to hours).
The slow heating rates and extended processing time are designed to maximize the production of the solid residue, biochar. This process is often favored for applications in agriculture, soil amendment, and carbon sequestration.
Fast Pyrolysis: Maximizing Bio-oil
Fast pyrolysis aims to produce liquid bio-oil, a potential biofuel. It operates at moderate temperatures (around 450-600°C) but requires extremely rapid heating and a very short vapor residence time, typically less than two seconds.
The engineering challenge here is to heat the feedstock quickly and then immediately quench the resulting vapors to prevent them from breaking down into gas. This maximizes the liquid yield, which can be up to 75% by weight.
Flash Pyrolysis: A Higher-Energy Variant
Flash pyrolysis pushes the parameters further, with even higher temperatures (600-1000°C) and shorter residence times (often less than one second).
This high-energy process can be tuned to favor either bio-oil or, at the higher end of the temperature spectrum, the production of valuable chemical feedstocks and syngas.
Understanding the Trade-offs
Choosing a pyrolysis setup is an exercise in managing competing outcomes. You cannot maximize all products simultaneously.
The Product Yield Triangle: Char, Oil, Gas
Think of the products as corners of a triangle. By adjusting temperature and time, you move your process closer to one corner at the expense of the others.
- Low & Slow = Biochar
- Moderate & Fast = Bio-oil
- High & Fast/Slow = Syngas
Your desired output dictates the process conditions you must implement.
The Critical Role of Heating Rate
It is not just the final temperature that matters, but how quickly the feedstock reaches it. Fast and flash pyrolysis depend on very high heating rates to rapidly break down the material.
Slow pyrolysis, in contrast, uses very low heating rates (e.g., 1-30°C per minute). This technical parameter is a critical design consideration for any pyrolysis reactor.
Feedstock Is a Key Variable
The ideal temperature and residence time also depend heavily on the feedstock being processed. Woody biomass, agricultural waste, plastics, and tires all have different chemical compositions and will break down differently. The numbers provided here are a general guide, but precise optimization is always required for a specific material.
Choosing the Right Process for Your Goal
To select the correct parameters, start by defining your primary objective.
- If your primary focus is carbon sequestration or soil amendment: Use slow pyrolysis (400-500°C, hours of residence time) to maximize your yield of stable biochar.
- If your primary focus is creating a liquid biofuel or chemical feedstock: Use fast pyrolysis (450-600°C, <2 seconds residence time) to maximize your yield of bio-oil.
- If your primary focus is generating energy from gas: Use high-temperature pyrolysis or gasification (>700°C) to convert the feedstock primarily into flammable syngas.
Mastering the interplay of temperature and time is the fundamental skill required to unlock the full potential of pyrolysis.

Summary Table:
| Pyrolysis Type | Temperature Range (°C) | Residence Time | Primary Product |
|---|---|---|---|
| Slow Pyrolysis | 400 - 500 | Hours | Biochar |
| Fast Pyrolysis | 450 - 600 | < 2 seconds | Bio-oil |
| Flash Pyrolysis | 600 - 1000 | < 1 second | Syngas / Chemicals |
Ready to optimize your pyrolysis process? KINTEK specializes in high-performance lab reactors and pyrolysis equipment designed for precise temperature control and residence time management. Whether your goal is maximizing biochar for carbon sequestration, bio-oil for fuel, or syngas for energy, our solutions deliver reliable, scalable results. Contact our experts today to discuss your specific feedstock and product goals!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How do you measure ash content? Choose the Right Method for Accurate Results
- Can calcination be done in a muffle furnace? Yes, for precise air-atmosphere heating.
- What precautions should be taken while heating and cooling the crucible? Prevent Thermal Shock and Ensure Safety
- How do you test a metal to determine its quality? Verify Mechanical & Chemical Properties for Your Application
- How do you check the ash content of a muffle furnace? A Step-by-Step Guide to Material Analysis



















