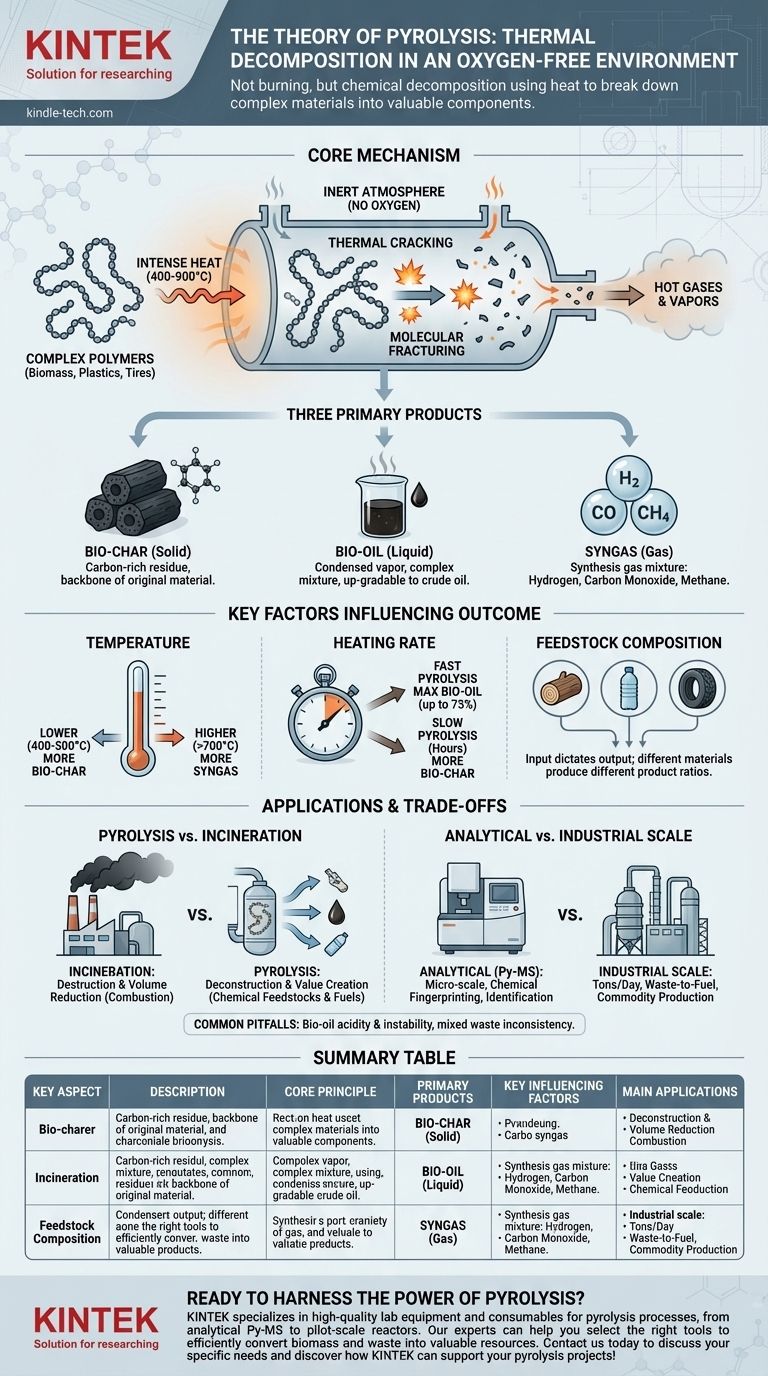
At its core, pyrolysis is the thermal decomposition of materials at high temperatures in an oxygen-free environment. Instead of burning, which is a combustion reaction with oxygen, the intense heat alone fractures the material's complex molecules into a mixture of smaller, simpler substances. This controlled deconstruction process yields three distinct products: a solid residue (bio-char), a liquid (bio-oil), and a gas (syngas).
Pyrolysis is not burning; it is chemical decomposition using heat. By controlling the temperature and eliminating oxygen, we can precisely break down complex organic materials into valuable, reusable components like gas, liquid, and a charcoal-like solid.
The Core Mechanism: How Pyrolysis Works
The Critical Role of an Inert Atmosphere
The defining characteristic of pyrolysis is the absence of oxygen. When oxygen is present, high heat causes combustion—a rapid chemical reaction that releases energy as heat and light, producing primarily carbon dioxide and water.
By conducting the process in an inert atmosphere (like nitrogen) or a vacuum, combustion is prevented. This forces the heat energy to be absorbed directly by the chemical bonds within the material, causing them to break apart.
Thermal Cracking at the Molecular Level
The materials best suited for pyrolysis—such as biomass, plastics, or tires—are made of large, long-chain molecules called polymers. The high heat (typically 400-900°C) provides the activation energy needed to sever these long chains.
This process, known as thermal cracking, breaks the large, non-volatile molecules into smaller, lighter, and more volatile fragments. These fragments leave the solid material as hot gases and vapors.
The Three Primary Products
As the hot gases and vapors are processed, they are separated into the final three outputs.
- Bio-char (Solid): This is the carbon-rich solid residue that remains after all volatile components have been driven off. It is the backbone of the original material, stripped of everything else.
- Bio-oil (Liquid): When the hot vapor stream is cooled rapidly, a significant portion condenses into a liquid known as pyrolysis oil or bio-oil. This complex mixture of compounds can be upgraded into a form of crude oil.
- Syngas (Gas): The remaining components that do not condense into a liquid form a mixture of gases. Known as synthesis gas or syngas, it is primarily composed of hydrogen, carbon monoxide, and methane.
Key Factors Influencing the Outcome
The precise ratio and composition of the three end products are not accidental. They can be carefully controlled by manipulating the pyrolysis conditions.
Temperature
Temperature is a primary lever. Lower temperatures (around 400-500°C) and slower heating tend to maximize the yield of bio-char. Conversely, higher temperatures (above 700°C) favor the production of syngas.
Heating Rate
The speed at which the material is heated also has a dramatic effect. A process known as fast pyrolysis, which heats the material to temperature in seconds, maximizes the yield of liquid bio-oil, often making up 75% of the product by weight. Slow pyrolysis, which can take hours, yields more bio-char.
Feedstock Composition
The chemical makeup of the input material, or feedstock, directly dictates the output. Pyrolysis of wood biomass will produce a different bio-oil and char than the pyrolysis of plastic waste or old tires. Understanding the feedstock is crucial for predicting and managing the results.
Understanding the Trade-offs and Applications
Pyrolysis is not a single technique but a fundamental principle with applications ranging from laboratory analysis to industrial-scale processing.
Pyrolysis vs. Incineration
It is critical to distinguish pyrolysis from incineration. Incineration is a process of destruction aimed at volume reduction and simple heat recovery. Pyrolysis is a process of deconstruction aimed at creating valuable chemical feedstocks and fuels.
Analytical vs. Industrial Scale
On a micro-scale, Pyrolysis-Mass Spectrometry (Py-MS) uses the exact same principle. A tiny sample is pyrolyzed, and the resulting fragments are fed into a mass spectrometer to create a unique chemical "fingerprint," allowing for precise material identification.
On an industrial scale, large pyrolysis plants process tons of waste per day, turning municipal waste, agricultural residue, or end-of-life plastics into fuel and other valuable commodities.
Common Pitfalls to Avoid
The primary challenge in commercial pyrolysis is the complexity of the outputs. Bio-oil is typically acidic, corrosive, and unstable, often requiring significant secondary processing (or "upgrading") before it can be used as a drop-in fuel. Likewise, managing mixed waste feedstock can lead to inconsistent product quality, which is a major engineering hurdle.
Making the Right Choice for Your Goal
The value of pyrolysis lies in its versatility. How you apply it depends entirely on your objective.
- If your primary focus is materials science: View pyrolysis as a precise analytical method (Py-MS) to fingerprint and understand the chemical composition of complex polymers.
- If your primary focus is waste management: See pyrolysis as a powerful alternative to landfills and incineration, converting problematic waste streams into valuable resources.
- If your primary focus is renewable energy: Recognize pyrolysis as a key technology for producing biofuels (bio-oil and syngas) and carbon-sequestering products (bio-char) from biomass.
Understanding the theory of pyrolysis allows you to see it not as a simple disposal method, but as a sophisticated tool for chemical transformation.

Summary Table:
| Key Aspect | Description |
|---|---|
| Core Principle | Thermal decomposition of materials in an oxygen-free environment. |
| Primary Products | Bio-char (solid), Bio-oil (liquid), Syngas (gas). |
| Key Influencing Factors | Temperature, Heating Rate, Feedstock Composition. |
| Main Applications | Waste management, Renewable energy production, Analytical science (Py-MS). |
Ready to harness the power of pyrolysis in your lab or facility? KINTEK specializes in high-quality lab equipment and consumables for pyrolysis processes, from analytical Py-MS to pilot-scale reactors. Our experts can help you select the right tools to efficiently convert biomass and waste into valuable resources. Contact us today to discuss your specific needs and discover how KINTEK can support your pyrolysis projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















