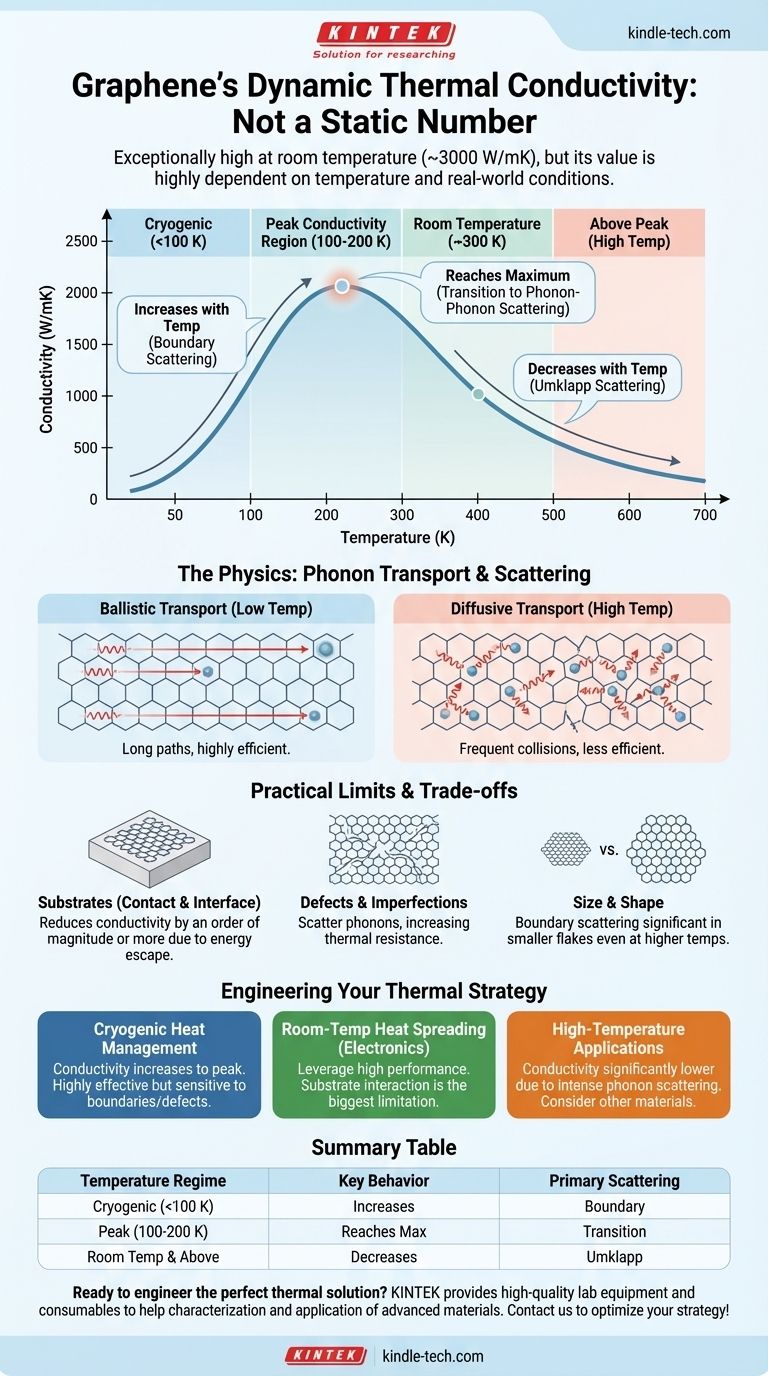
At room temperature, graphene exhibits exceptionally high thermal conductivity, but its behavior is highly dependent on temperature. This value is not static; it generally peaks at low temperatures and then decreases as temperature rises due to changes in how heat carriers, known as phonons, travel through its lattice. For ideal, suspended single-layer graphene, room-temperature conductivity can exceed 3000 W/mK, far surpassing materials like copper or diamond.
The thermal conductivity of graphene is not a single number but a dynamic property dictated by temperature. Its exceptional ability to conduct heat stems from the behavior of phonons, and understanding how these heat carriers scatter is the key to predicting graphene's performance in any real-world application.
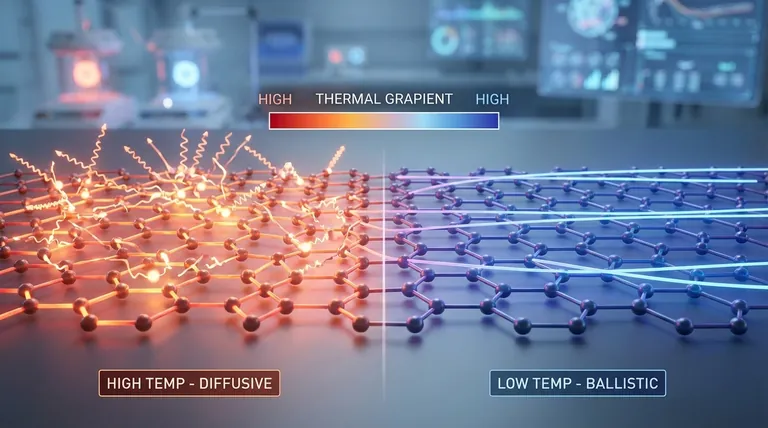
The Physics of Heat Conduction in Graphene
The Central Role of Phonons
Heat in a solid material like graphene is transported primarily by phonons, which are quantized packets of vibrational energy that travel through the crystal lattice.
The efficiency of this heat transport—its thermal conductivity—is determined by how freely these phonons can move before being scattered.
Ballistic vs. Diffusive Transport
At very low temperatures, phonons can travel long distances without interruption, a state known as ballistic transport.
As temperature increases, phonons become more numerous and begin to scatter off each other. This transforms the flow into diffusive transport, which is less efficient and results in lower thermal conductivity.
How Temperature Governs Graphene's Conductivity
The relationship between graphene's thermal conductivity and temperature can be understood by examining different temperature regimes.
At Very Low Temperatures (Cryogenic)
In the cryogenic range (below ~100 K), the number of phonons is low, and they do not scatter off each other frequently.
Instead, the main limiting factor is boundary scattering, where phonons collide with the physical edges of the graphene flake. In this regime, thermal conductivity actually increases with temperature as more vibrational modes become active.
The Peak Conductivity Region
Graphene's thermal conductivity reaches a peak at a specific low temperature (often between 100 K and 200 K).
This peak represents the transition point where scattering between phonons begins to dominate over boundary scattering as the primary resistance to heat flow.
At Room Temperature and Above
Above its peak, graphene's thermal conductivity consistently decreases as temperature rises.
This is due to a powerful type of phonon-phonon interaction called Umklapp scattering. As the lattice vibrates more intensely at higher temperatures, these scattering events become much more frequent, severely restricting the flow of heat.
Understanding the Practical Limits and Trade-offs
The theoretical values for graphene are impressive, but real-world performance is often much lower due to several factors that introduce new ways for phonons to scatter.
The Impact of Substrates
Most applications require placing graphene on a substrate (like silicon dioxide). This contact creates new pathways for vibrational energy to escape and introduces scattering at the interface.
A substrate can easily reduce graphene's effective thermal conductivity by an order of magnitude or more compared to its ideal, suspended state.
Defects, Wrinkles, and Grain Boundaries
Real-world graphene is not a perfect, infinite crystal. It contains defects, impurities, wrinkles, and grain boundaries.
Each of these imperfections acts as a scattering site for phonons, creating thermal resistance and lowering the overall conductivity.
The Role of Size and Shape
In smaller graphene flakes, boundary scattering remains a significant factor even at higher temperatures. The mean free path of the phonons can be limited by the physical dimensions of the material itself.
Applying This to Your Thermal Management Goal
Your engineering approach must account for this dynamic behavior. The optimal use of graphene depends entirely on the target operating temperature and material quality.
- If your primary focus is cryogenic heat management: Expect graphene's thermal conductivity to increase with temperature up to its peak, making it highly effective but also very sensitive to its physical boundaries and defects.
- If your primary focus is room-temperature heat spreading (e.g., in electronics): Leverage graphene's high performance, but recognize that its conductivity will decrease as the device heats up. Substrate interaction will likely be the single biggest limiting factor to address.
- If your primary focus is high-temperature applications: Understand that graphene's thermal conductivity will be significantly lower than its room-temperature value due to intense phonon-phonon scattering, potentially making other materials more suitable.
Ultimately, treating graphene's thermal conductivity as a dynamic system—not a static value—is the first step toward engineering effective thermal solutions.
Summary Table:
| Temperature Regime | Key Behavior | Primary Scattering Mechanism |
|---|---|---|
| Cryogenic (<100 K) | Increases with temperature | Boundary scattering |
| Peak (100-200 K) | Reaches maximum | Transition to phonon-phonon scattering |
| Room Temperature & Above | Decreases with temperature | Umklapp scattering |
Ready to engineer the perfect thermal solution for your application? Understanding graphene's dynamic conductivity is just the first step. At KINTEK, we specialize in providing high-quality lab equipment and consumables to help you characterize and apply advanced materials like graphene. Whether you're working on cryogenic systems, electronics cooling, or high-temperature processes, our expertise can support your R&D and production needs. Contact us today to discuss how we can help optimize your thermal management strategy!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Automatic Laboratory Heat Press Machine
- High Temperature Wear-Resistant Alumina Al2O3 Plate for Engineering Advanced Fine Ceramics
People Also Ask
- What is the temperature of a graphite furnace? Achieve Extreme Heat Up to 3000°C
- What are the advantages of graphite furnace? Achieve High-Temperature Precision and Purity
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- Why graphite is used in furnace? Achieve Superior Heat Treatment & Energy Efficiency