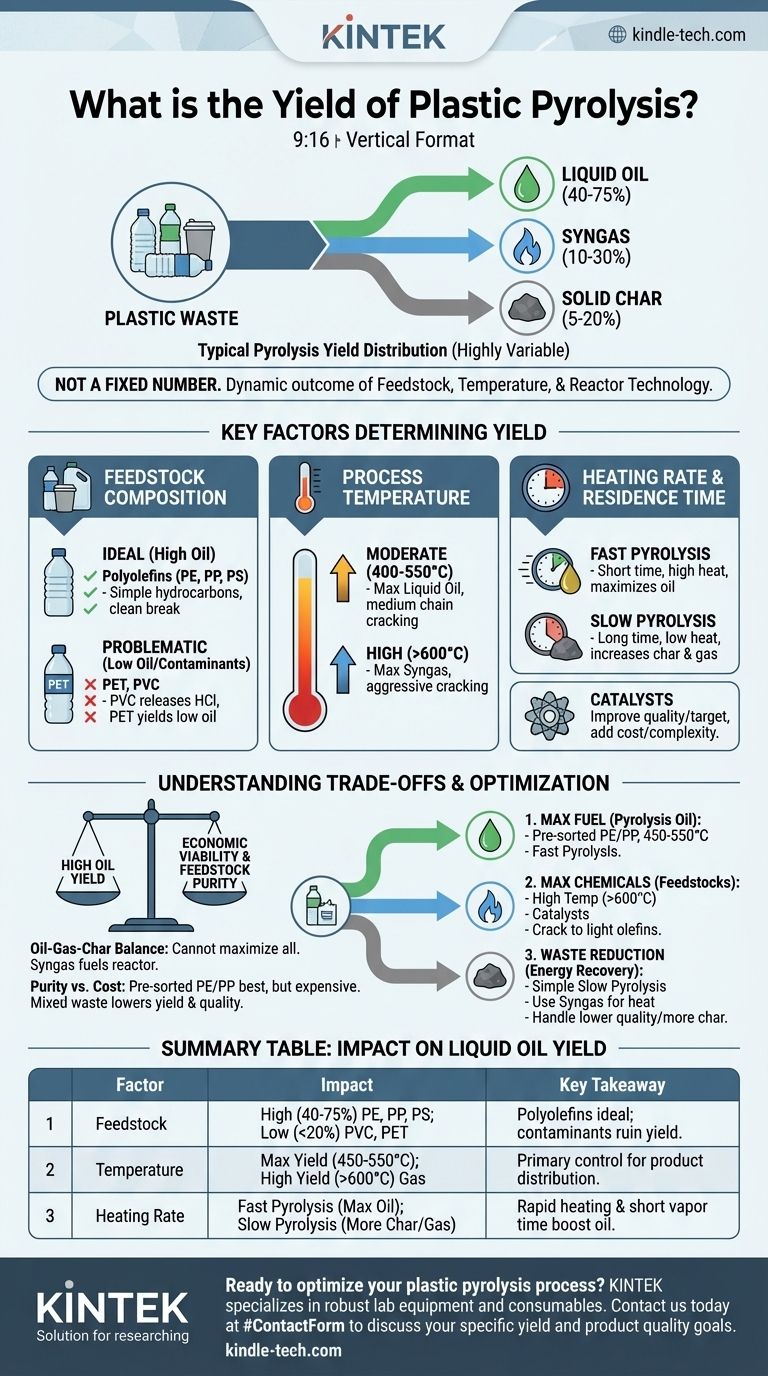
In short, the yield of liquid oil from plastic pyrolysis typically ranges from 40% to 75% by weight. The remaining products are a non-condensable synthetic gas (syngas), usually 10-30%, and a solid carbon residue, or char, at 5-20%. These figures are highly variable and not guaranteed.
The specific yield of any plastic pyrolysis system is not a fixed number. It is a dynamic outcome dictated by the type of plastic feedstock, the process temperature, and the reactor technology used. Understanding these variables is the key to assessing the true potential of the process.

Key Factors That Determine Pyrolysis Yield
Achieving a desirable yield is an exercise in chemical engineering control. The output is a direct consequence of the inputs and process conditions you select.
Feedstock Composition: The Starting Point
The type of plastic you put into the reactor is the single most important factor. Plastics are not all created equal.
Polyolefins (PE, PP, PS) like polyethylene, polypropylene, and polystyrene are ideal. They are simple hydrocarbon chains that break down cleanly into oil, gas, and char. These consistently produce the highest liquid oil yields.
Contaminant Plastics (PET, PVC) are highly problematic. Polyvinyl chloride (PVC) releases corrosive hydrochloric acid when heated, which can destroy equipment and contaminate the final oil. Polyethylene terephthalate (PET) yields very little oil, instead producing solid terephthalic acid and water, which lowers the overall process efficiency.
Process Temperature: The Primary Control Lever
Temperature directly controls how the long polymer chains break apart (a process called "cracking").
Moderate Temperatures (400–550°C) are the sweet spot for maximizing liquid oil. In this range, the polymer chains crack into medium-length hydrocarbon molecules that condense into a liquid oil at room temperature.
High Temperatures (>600°C) cause more aggressive secondary cracking. The medium-length oil molecules are broken down further into very short, light molecules. This dramatically increases the yield of non-condensable syngas at the expense of liquid oil.
Heating Rate and Residence Time: Fine-Tuning the Reaction
How quickly you heat the plastic and how long you keep it at temperature also shapes the output.
Fast Pyrolysis, characterized by a very high heating rate and short residence time (seconds), is designed to quickly vaporize the plastic and remove the vapors from the hot zone before they can over-react. This method maximizes the liquid oil yield.
Slow Pyrolysis, with a low heating rate and long residence time (minutes to hours), allows for secondary reactions to occur. This tends to increase the proportion of stable char and gas, reducing the final liquid yield.
The Role of Catalysts
Introducing a catalyst into the process can significantly alter the outcome. Catalysts, such as zeolites, can lower the required reaction temperature.
More importantly, they can selectively guide the cracking reactions to produce a higher-quality oil with a more desirable composition, such as hydrocarbons in the gasoline or diesel range. While this improves product value, it adds significant cost and complexity to the operation.
Understanding the Trade-offs
There is no "perfect" yield. Optimizing for one product often comes at the expense of another, and laboratory results rarely translate directly to industrial-scale economics.
The Oil-Gas-Char Balance
You cannot maximize all three outputs simultaneously. A process tuned for high oil yield will inherently produce a specific amount of gas and char. The non-condensable gas is not waste; it is typically captured and used as fuel to power the pyrolysis reactor, reducing external energy costs.
The Problem of Feedstock Purity
While pure polyolefin streams give the best results, real-world post-consumer plastic waste is heavily mixed and contaminated. The cost of sorting this waste to achieve a "clean" feedstock is a major economic hurdle. Running a mixed, un-sorted stream will result in lower oil yields, lower oil quality, and potential operational issues from materials like PVC and PET.
Economic Viability vs. Ideal Yield
The highest possible oil yield does not always equal the most profitable operation. A process with a slightly lower yield but significantly lower energy costs, no need for expensive catalysts, and the ability to handle less-pure feedstock may be far more economically viable in the long run.
Making the Right Choice for Your Goal
The "best" yield depends entirely on your objective. Use these guidelines to align the process with your desired outcome.
- If your primary focus is maximizing liquid fuel (pyrolysis oil): Use pre-sorted polyolefin feedstock (PE, PP), and run the process at moderate temperatures (450-550°C) with a reactor designed for fast pyrolysis.
- If your primary focus is producing valuable chemical feedstocks: Employ higher temperatures (>600°C) and catalysts to crack the polymers back into light olefins like ethylene and propylene for the chemical industry.
- If your primary focus is waste volume reduction with energy recovery: A simpler, slow pyrolysis process can be effective, but plan to use the significant syngas output for process heat and be prepared to handle a lower-quality oil and a higher volume of char.
Ultimately, optimizing pyrolysis yield is a balancing act between feedstock purity, process control, and your specific economic or environmental objective.
Summary Table:
| Factor | Impact on Liquid Oil Yield | Key Takeaway |
|---|---|---|
| Feedstock (Plastic Type) | High (40-75%): Pure PE, PP, PS Low (<20%): PVC, PET |
Polyolefins are ideal; contaminants ruin yield and equipment. |
| Process Temperature | Max Yield (450-550°C): Optimal for oil High Yield (>600°C): Favors gas production |
Temperature is the primary control for product distribution. |
| Heating Rate & Time | Fast Pyrolysis: Maximizes oil Slow Pyrolysis: Increases char and gas |
Rapid heating and short vapor residence time boost oil output. |
| Use of Catalyst | Can increase quality and target specific hydrocarbons. | Adds cost and complexity but can improve product value. |
Ready to optimize your plastic pyrolysis process for maximum yield and efficiency? The figures above are highly variable and depend on precise control of feedstock and reactor conditions. KINTEK specializes in providing robust lab equipment and consumables for pyrolysis research and development. Whether you are testing feedstock purity, optimizing temperature profiles, or scaling your process, our tools deliver the accuracy and reliability you need.
Contact us today at #ContactForm to discuss how our solutions can help you achieve your specific yield and product quality goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds



















