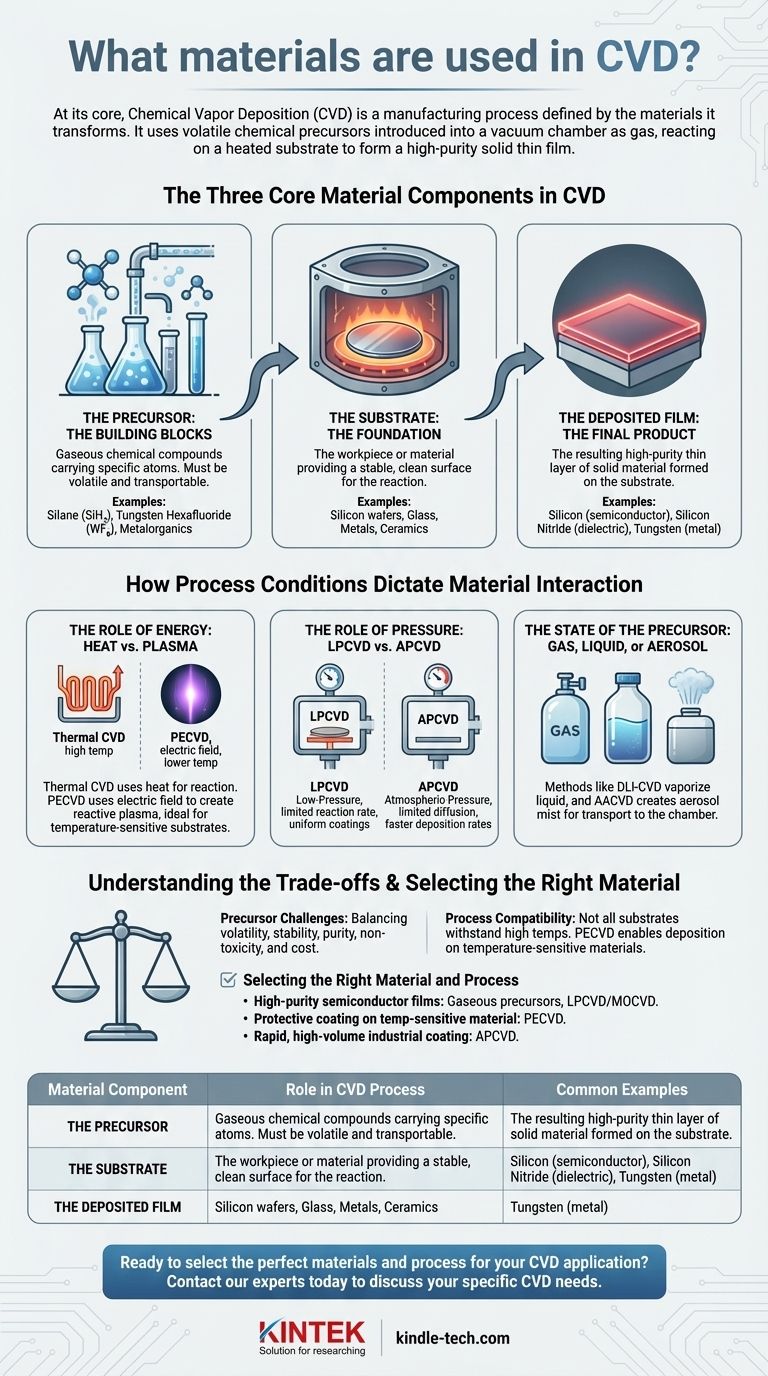
At its core, Chemical Vapor Deposition (CVD) is a manufacturing process defined by the materials it transforms. The process uses volatile chemical compounds, known as precursors, which are introduced into a vacuum chamber as a gas. These precursors react and decompose on a heated surface, or substrate, leaving behind a high-purity solid thin film of the desired material.
The choice of materials in CVD is a strategic decision that dictates the entire process. The precursor chemical, the substrate foundation, and the specific CVD method (e.g., thermal or plasma-based) are interconnected variables that determine the final properties of the deposited layer.

The Three Core Material Components in CVD
To understand CVD, you must first understand the three key materials involved in every deposition.
The Substrate: The Foundation
The substrate is the workpiece or material onto which the thin film is deposited. Its primary role is to provide a stable, clean surface for the chemical reaction to occur.
The choice of substrate is critical, as it must be able to withstand the temperature and chemical environment of the CVD process. Common substrates include silicon wafers, glass, metals, and ceramics.
The Precursor: The Building Blocks
Precursors are the gaseous chemical compounds that carry the specific atoms you want to deposit. They are the fundamental building blocks of the final film.
These materials must be volatile enough to be transported in a gaseous state but stable enough not to decompose before reaching the substrate. They can be sourced from gases, vaporized liquids, or sublimated solids.
The Deposited Film: The Final Product
The deposited film is the resulting thin layer of solid material formed on the substrate. The properties of this film are the entire goal of the process.
The type of film can be anything from a semiconductor (like silicon), a dielectric insulator (like silicon nitride), or a conductive metal (like tungsten), depending entirely on the precursor chemicals used.
How Process Conditions Dictate Material Interaction
The specific type of CVD process used is chosen based on the properties of the precursor and substrate materials. The references highlight several key process variables that control how these materials interact.
The Role of Energy: Heat vs. Plasma
A chemical reaction requires energy. In thermal CVD, this energy is supplied by heating the substrate to very high temperatures, causing the precursor gases to react and deposit material.
In Plasma-Enhanced CVD (PECVD), this energy is supplied by an electric field that ignites a plasma. This plasma creates highly reactive chemical species without requiring extremely high temperatures, making it ideal for temperature-sensitive substrates.
The Role of Pressure: LPCVD vs. APCVD
Pressure controls how the precursor gas molecules travel to the substrate surface.
In Low-Pressure CVD (LPCVD), the reaction is limited by the rate of the chemical reaction on the surface itself. This results in highly uniform, conformal coatings.
In Atmospheric-Pressure CVD (APCVD), the chamber is at normal pressure. Here, the process is limited by how fast the gas can diffuse to the surface (mass transfer), which allows for much faster deposition rates.
The State of the Precursor: Gas, Liquid, or Aerosol
While many precursors are gases at room temperature, others are liquids or solids. Methods like Direct Liquid Injection (DLI-CVD) vaporize a liquid precursor just before it enters the chamber.
Similarly, Aerosol-Assisted CVD (AACVD) dissolves the precursor in a solvent and creates a fine mist, or aerosol, that is then transported to the reaction chamber.
Understanding the Trade-offs
Selecting the right materials and process involves balancing competing factors. What works for one application may be entirely unsuitable for another.
Precursor Selection Challenges
The ideal precursor is highly volatile, stable, pure, non-toxic, and inexpensive. In reality, no precursor meets all these criteria. A highly effective chemical might be dangerously toxic or prohibitively expensive, forcing a compromise.
Process and Material Compatibility
Not all substrates can survive the high temperatures (often >600°C) of traditional thermal CVD. This is the primary reason plasma-based methods were developed—to enable the deposition of high-quality films on materials like plastics that would otherwise melt.
Purity and Contamination
The purity of the precursor gases is paramount. Any impurity within the precursor supply can be incorporated directly into the final film, potentially compromising its electrical, optical, or mechanical properties.
Selecting the Right Material and Process
Your end goal determines the optimal combination of materials and process conditions.
- If your primary focus is high-purity, uniform semiconductor films: You will likely use high-purity gaseous precursors like silane or metalorganics in a Low-Pressure CVD (LPCVD) or Metalorganic CVD (MOCVD) system.
- If your primary focus is depositing a protective coating on a temperature-sensitive material: You should consider Plasma-Enhanced CVD (PECVD), which uses plasma to enable reactions at much lower temperatures.
- If your primary focus is rapid, high-volume industrial coating: Atmospheric Pressure CVD (APCVD) is often suitable, as its mass-transfer-limited nature allows for faster deposition rates.
Understanding the interplay between the precursor, the substrate, and the process energy is the key to mastering CVD for any application.
Summary Table:
| Material Component | Role in CVD Process | Common Examples |
|---|---|---|
| Precursor | Gaseous chemical compound carrying atoms for deposition; the 'building block'. | Silane (SiH₄), Tungsten Hexafluoride (WF₆), Metalorganics |
| Substrate | The foundation or workpiece onto which the thin film is deposited. | Silicon wafers, Glass, Metals, Ceramics |
| Deposited Film | The final, high-purity solid layer formed on the substrate. | Silicon (semiconductor), Silicon Nitride (dielectric), Tungsten (metal) |
Ready to select the perfect materials and process for your CVD application?
At KINTEK, we specialize in providing the lab equipment and consumables you need to master Chemical Vapor Deposition. Whether you are developing high-purity semiconductor films with LPCVD, coating temperature-sensitive materials with PECVD, or scaling up with APCVD, our expertise and products support your success.
We understand that the right combination of precursor, substrate, and process is critical. Let us help you achieve the precise, high-quality thin films your research or production demands.
Contact our experts today to discuss your specific CVD needs and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond Cutting Tool Blanks for Precision Machining
- CVD Diamond Domes for Industrial and Scientific Applications
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- How does plasma vapor deposition work? A Low-Temperature Coating Solution for Sensitive Materials
- What is plasma CVD? Unlock Low-Temperature Thin Film Deposition for Sensitive Materials
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is plasma enhanced? A Guide to Low-Temperature, High-Precision Manufacturing



















