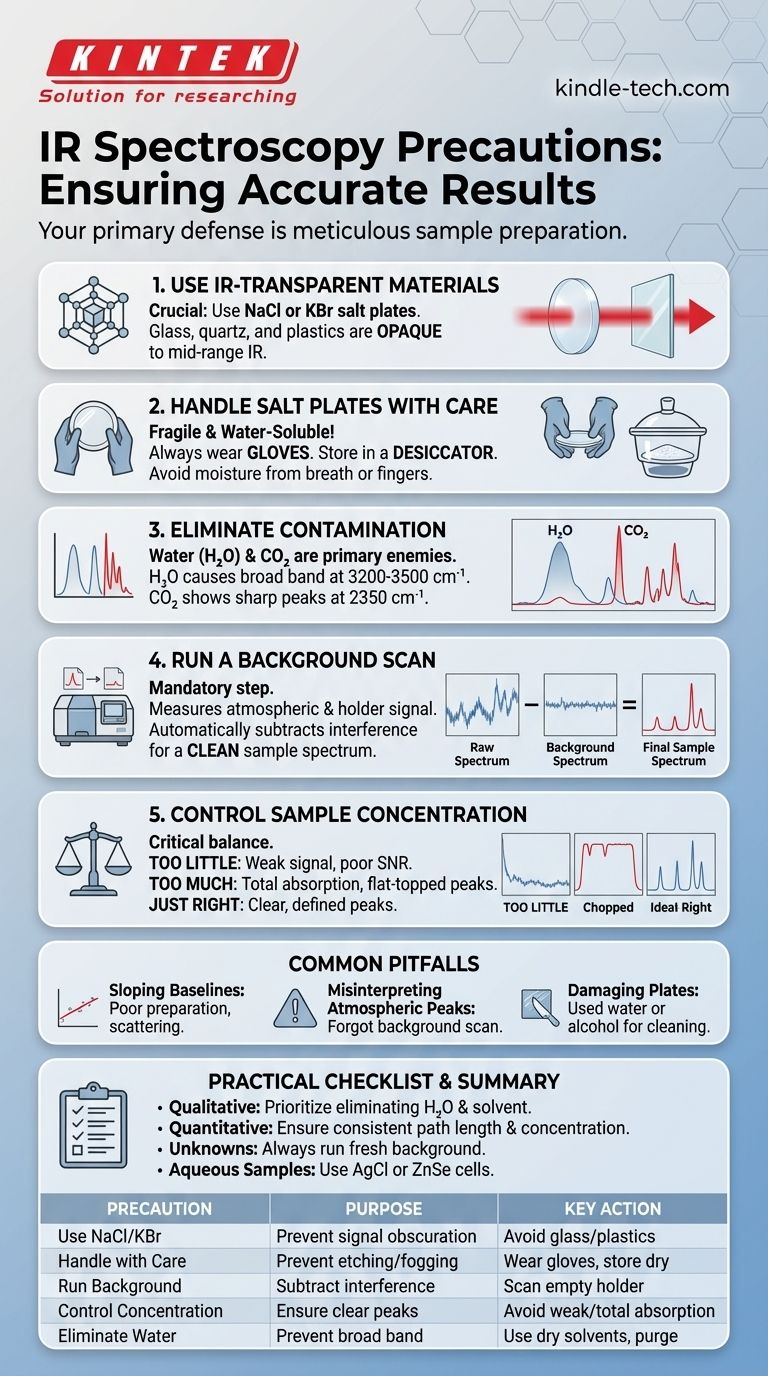
To ensure accurate IR spectroscopy results, your most critical precautions must focus on sample preparation. This involves using materials that are transparent to infrared radiation, such as sodium chloride (NaCl) or potassium bromide (KBr) salt plates, and meticulously ensuring your sample is pure and properly concentrated to avoid distorted or misleading data.
The core principle behind every IR spectroscopy precaution is to ensure the spectrum you record is only from your sample. Any other substance that absorbs IR radiation—including the sample holder, solvents, or atmospheric moisture—will contaminate the data and lead to incorrect interpretations.

Why Sample Preparation Dictates Success
Infrared spectroscopy works by passing IR radiation through a sample and measuring which frequencies are absorbed. If the container holding the sample also absorbs IR radiation, its signal will overlap with and obscure the signal from your substance of interest.
The Necessity of IR-Transparent Materials
Standard lab materials like glass, quartz, and most plastics are opaque to mid-range infrared radiation because their molecular bonds absorb it strongly. They cast a "shadow," blocking the instrument's view of your sample.
This is why alkali halide salts (NaCl, KBr) are used. Their simple ionic bonds do not absorb IR radiation in the typical analytical range (4000-400 cm⁻¹), making them effectively invisible to the spectrometer.
Proper Handling of Salt Plates
Salt plates are the windows through which the IR beam passes. However, they are fragile and require specific handling.
Because they are made of salt, they are highly soluble in water. Even moisture from your breath or fingers can etch and fog the plates, rendering them useless. Always handle them with gloves and store them in a desiccator to keep them dry.
Key Precautions for Accurate Spectra
Meaningful data with sharp, well-defined peaks comes from eliminating all sources of interference. Your preparation technique is the primary line of defense.
Avoid Contamination at All Costs
The most common contaminant in IR spectroscopy is water (H₂O). Moisture from the atmosphere, solvents, or the sample itself will produce a very broad absorption band around 3200-3500 cm⁻¹, which can easily mask important O-H or N-H signals from your actual sample.
Another common contaminant is **carbon dioxide (CO₂) ** from the air, which shows sharp peaks around 2350 cm⁻¹. High-end instruments are often purged with dry nitrogen to eliminate both H₂O and CO₂ interference.
Run a Background Scan
Before running your sample, you must run a background spectrum. This scan measures the signal from the instrument's environment (e.g., atmospheric CO₂ and H₂O) and the empty sample holder (e.g., the salt plates).
The instrument then automatically subtracts this background from your sample's spectrum. This crucial step ensures the final result shows absorptions from the sample alone.
Control Sample Concentration
The amount of sample in the IR beam's path is critical.
- Too little sample results in weak signals and a poor signal-to-noise ratio, making small peaks impossible to identify.
- Too much sample causes "total absorption." The peaks will appear flat-topped because all the light at those frequencies is blocked. This makes the data quantitatively useless and can hide the true peak shape.
Common Pitfalls and How to Avoid Them
Improper technique can introduce artifacts into your spectrum that look like real data but are actually errors in preparation or measurement.
The Problem of Sloping Baselines
An ideal spectrum has a flat baseline at or near 100% transmittance. A sloping baseline is often caused by a poorly prepared sample that scatters the IR light, such as a KBr pellet that is cloudy or a liquid film that has an inconsistent thickness. This scattering makes it difficult to determine accurate peak intensities.
Misinterpreting Atmospheric Peaks
Forgetting to run a fresh background scan can lead to sharp peaks for atmospheric CO₂ or rolling waves from H₂O vapor appearing in your spectrum. An analyst who is not careful might mistakenly assign these artifacts to their sample.
Damaging Salt Plates with Solvents
Never use water or alcohols to clean salt plates, as they will dissolve. Clean them with a dry, non-polar solvent like anhydrous dichloromethane or hexane and immediately return them to a desiccator.
A Practical Checklist for Reliable Spectra
Your specific goal determines which precautions are most critical.
- If your primary focus is qualitative identification ("What is this?"): Your main goal is a clean spectrum. Prioritize the complete elimination of water and residual solvent contamination above all else.
- If your primary focus is quantitative analysis ("How much is there?"): You must ensure a consistent path length and prepare samples with a concentration that keeps your key analytical peaks below total absorption.
- If you are analyzing an unknown sample: Always run a background spectrum immediately before your sample scan to minimize errors from changing atmospheric conditions.
- If you are working with a sample containing water: Consider using specialized water-resistant sample cells, such as those made from AgCl or ZnSe, instead of standard salt plates.
Following these fundamental precautions transforms IR spectroscopy from a routine measurement into a powerful and precise analytical tool.
Summary Table:
| Precaution | Purpose | Key Action |
|---|---|---|
| Use IR-Transparent Materials (NaCl, KBr) | Ensure sample signal is not obscured | Avoid glass, quartz, plastics |
| Handle Salt Plates with Care | Prevent etching and fogging | Wear gloves, store in desiccator |
| Run a Background Scan | Subtract atmospheric interference (H₂O, CO₂) | Scan empty holder before sample |
| Control Sample Concentration | Avoid weak signals or total absorption | Adjust for clear, defined peaks |
| Eliminate Water Contamination | Prevent broad O-H band interference | Use dry solvents, purge with N₂ |
Need precise IR spectroscopy results? KINTEK specializes in lab equipment and consumables, providing the high-quality IR-transparent materials and accessories essential for accurate sample preparation. From durable salt plates to reliable sample holders, our products help you eliminate contamination and achieve clean, interpretable spectra. Let our experts support your laboratory's analytical needs—contact us today to enhance your IR spectroscopy workflow!
Visual Guide
Related Products
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Hexagonal Boron Nitride HBN Ceramic Ring
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
People Also Ask
- What is the function of an ultra-high pressure hydraulic press in ceramic composites? Achieving High-Density Densification
- What is the purpose of using stainless steel molds and laboratory hydraulic presses? Ensure Precise Ionic Conductivity
- What are the risks of a hydraulic press? Essential Safety and Operational Insights
- Why is a laboratory hydraulic press essential for dry electrode films? Unlock High-Loading Battery Innovation
- What are press machines used for? Shaping, Bonding, and Compressing Materials with Precision
- What are the parts of a manual hydraulic press? A Guide to Its Core Components and Operation
- What are the three 3 differences between compression molding and injection molding? Choose the Right Process for Your Project
- What is the importance of KBr? The Essential Role in Spectroscopy & Medicine



















