Executing an electrolysis experiment safely is a matter of systematic preparation, not just last-minute checks. The essential precautions involve ensuring proper ventilation to handle potentially harmful gases, implementing rigorous electrical safety to prevent shock, using appropriate Personal Protective Equipment (PPE) to avoid chemical contact, and carefully managing the physical setup to prevent burns or pressure-related accidents.
True experimental safety is not a checklist of rules, but a mindset. It requires understanding that every electrolysis setup creates a confluence of chemical, electrical, and physical hazards that must be identified and actively managed before the experiment begins.

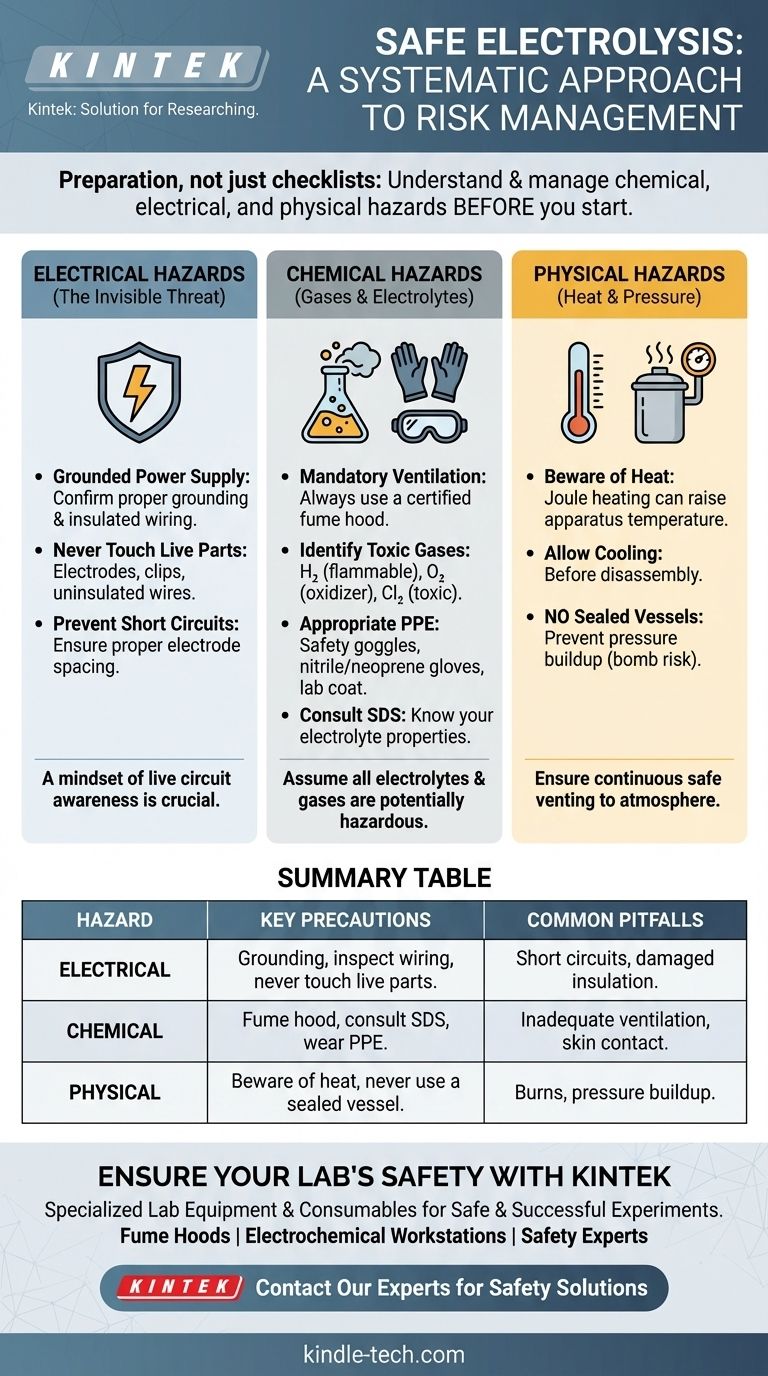
Deconstructing the Risks: A Three-Part Framework
A robust safety plan addresses three distinct categories of hazards inherent to any electrolysis process. Ignoring any one of these can lead to serious incidents.
Electrical Hazards: The Invisible Threat
The power supply is the most immediate and potentially lethal danger. An electric current sufficient for electrolysis is more than sufficient to cause severe injury or death.
Always confirm that your electrochemical workstation or power supply is properly grounded. Ensure all wiring is insulated, undamaged, and connected securely to the correct terminals before energizing the system.
Most importantly, never touch the electrodes, connecting clips, or uninsulated wires while the power is on. Treat every part of the circuit as live.
Chemical Hazards: Gases and Electrolytes
The core process of electrolysis breaks down chemical compounds, often creating new, more hazardous substances.
The gases generated at the electrodes can be a primary concern. For example, the electrolysis of water produces flammable hydrogen and an oxidizer (oxygen), creating an explosive mixture. The electrolysis of brine (salt water) can produce toxic chlorine gas.
For this reason, all electrolysis experiments should be conducted in a certified fume hood or a space with demonstrably high-rate ventilation. This is not optional.
The electrolyte itself also poses a risk. Many are corrosive acids or caustic bases. Always consult the Safety Data Sheet (SDS) for your electrolyte and wear appropriate PPE, including safety goggles, nitrile or neoprene gloves, and a lab coat, to prevent chemical burns and poisoning.
Physical Hazards: Heat and Pressure
Passing current through an electrolyte with internal resistance generates significant heat (Joule heating). This can raise the temperature of the entire apparatus.
Be aware that the beaker or reaction vessel can become hot enough to cause burns. Allow the system to cool before disassembly.
Crucially, never conduct electrolysis in a completely sealed vessel. The continuous production of gas will cause a rapid pressure increase, turning your apparatus into a potential bomb. The system must always be able to vent to the atmosphere safely.
Common Oversights and Pitfalls
Trust is built on acknowledging what can go wrong. Many incidents occur not from novel dangers, but from overlooking these common setup errors.
Inadequate Ventilation
A common mistake is assuming an open window provides sufficient ventilation. For any reactants or products that are toxic or flammable (like chlorine or hydrogen), this is a dangerous assumption. A fume hood is the professional standard for containing and removing these gases.
Improper Equipment Setup
The reference to correct electrode installation is critical. If electrodes are spaced too closely or shift during the experiment, they can touch, creating a short circuit. This can damage your power supply and create a significant fire hazard.
Furthermore, ensure only the electrode material is submerged. If the alligator clips or connecting rods are immersed in the electrolyte, they will corrode, contaminate your experiment, and potentially fail.
Underestimating the Electrolyte
Do not assume an electrolyte is harmless. Even a simple sodium chloride solution can produce hazardous chlorine gas. Never make direct skin contact with any electrolyte, and always handle them with the appropriate PPE.
Applying This to Your Experiment
Your specific safety focus will shift based on the context of your work. Use this framework to build your protocol.
- If your primary focus is a research lab experiment: Your priority is a formal risk assessment. Document every chemical, verify the fume hood certification, and ensure your electrochemical workstation is calibrated and electrically sound.
- If you are conducting a classroom demonstration: Your goal is containment and simplicity. Use low voltages, dilute and well-understood electrolytes (like a sodium sulfate solution), and maintain a clear physical barrier between the apparatus and the students.
- If your focus is scaling up for industrial application: Engineered controls are paramount. This includes automated temperature and pressure monitoring, emergency shutdown systems, and robust heat dissipation and gas management infrastructure.
A systematic approach to safety transforms potential hazards into controlled variables, ensuring both successful results and personal well-being.
Summary Table:
| Hazard Category | Key Precautions | Common Pitfalls |
|---|---|---|
| Electrical | Proper grounding, inspect wiring, never touch live parts. | Short circuits, damaged insulation. |
| Chemical | Use a fume hood, consult SDS, wear gloves/goggles/lab coat. | Inadequate ventilation, skin contact with electrolytes. |
| Physical | Beware of heat (Joule heating), never use a sealed vessel. | Burns from hot apparatus, pressure buildup. |
Ensure Your Lab's Electrolysis Safety with KINTEK
Electrolysis experiments demand precision and, above all, safety. The right equipment is your first line of defense against chemical, electrical, and physical hazards.
KINTEK specializes in the lab equipment and consumables that keep your experiments safe and successful. From certified fume hoods for containing hazardous gases to reliable electrochemical workstations with proper safety features, we provide the tools you need to manage risks effectively.
Let us help you build a safer lab. Our experts can assist you in selecting the right equipment for your specific application, whether it's for academic research, classroom demonstrations, or industrial scale-up.
Ready to enhance your lab's safety protocol? Contact our team today to discuss your needs and ensure your experiments are protected from start to finish.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
People Also Ask
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility



















