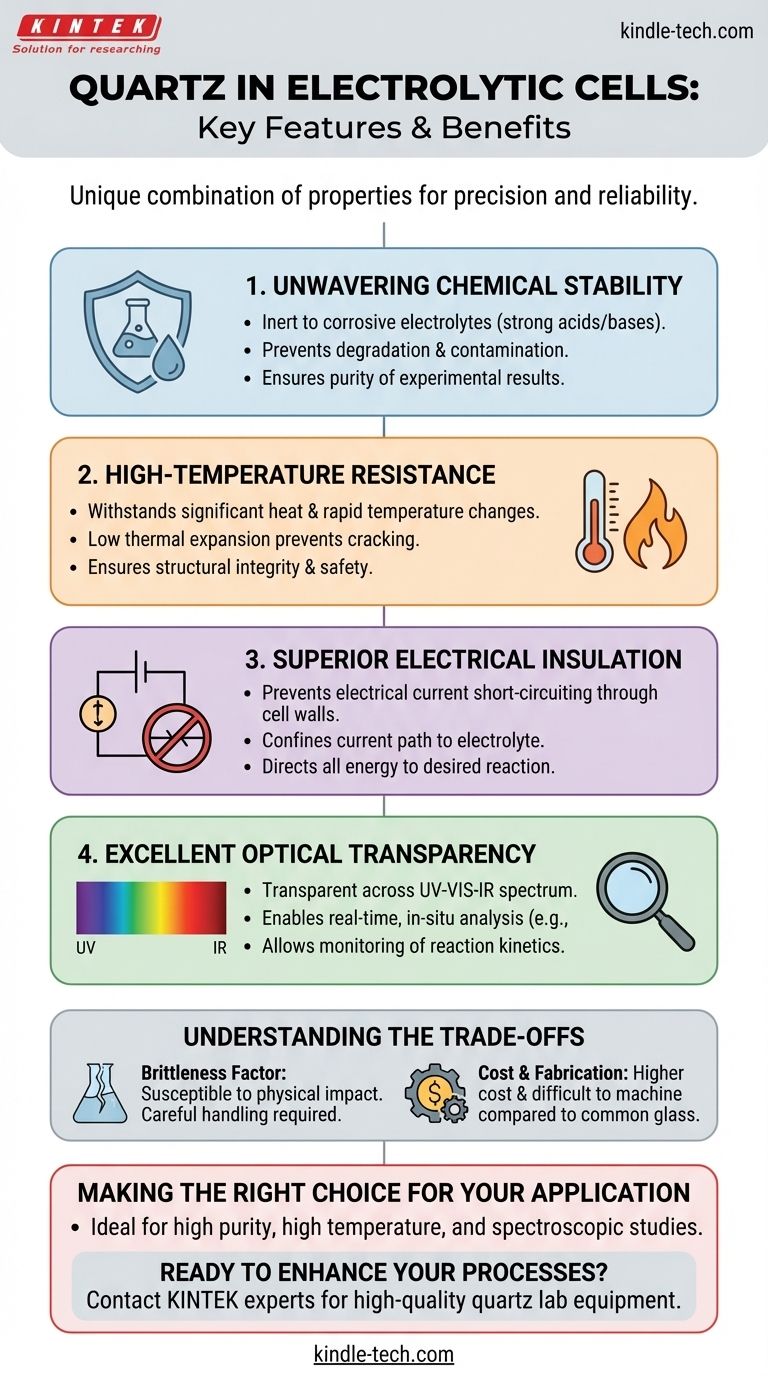
The suitability of quartz for electrolytic cells stems from a unique combination of four key properties. It possesses exceptional chemical stability, high-temperature resistance, excellent electrical insulation, and high optical transparency, making it a superior material for demanding electrochemical applications where precision and reliability are paramount.
Quartz is not merely a container for an electrochemical reaction; it is an inert, stable, and non-interfering environment that ensures the integrity and purity of the process, even under extreme operating conditions.

The Four Pillars of Quartz in Electrochemistry
To understand why quartz is so valued, we must examine how each of its core properties solves a specific challenge inherent in the design and operation of an electrolytic cell.
Unwavering Chemical Stability
Electrolytic cells frequently operate with highly corrosive electrolytes, such as strong acids or bases.
Quartz, being composed of high-purity silicon dioxide (SiO₂), is exceptionally inert. It does not react with these chemicals, preventing the cell walls from degrading and, just as importantly, stopping impurities from leaching into the solution.
This stability guarantees that the experimental results are a true reflection of the intended electrochemical reaction, free from contamination.
High-Temperature Resistance
Electrolysis can generate significant heat due to the electrical resistance of the electrolyte (Joule heating).
Quartz has a very high melting point and a very low coefficient of thermal expansion. This means it can withstand high operating temperatures and rapid temperature changes without cracking or deforming.
This thermal resilience ensures the structural integrity and safety of the cell during high-energy processes.
Superior Electrical Insulation
The entire principle of electrolysis relies on a controlled flow of electrical current through the electrolyte between two electrodes.
Quartz is an excellent electrical insulator. This property is critical because it prevents the current from "short-circuiting" through the walls of the cell.
By confining the electrical path to the electrolyte, quartz ensures that all the applied energy is directed exclusively to driving the desired chemical reaction.
Excellent Optical Transparency
Many advanced electrochemical studies rely on observing the reaction as it happens.
Quartz is transparent across a wide range of the electromagnetic spectrum, from ultraviolet (UV) to infrared (IR).
This high light transmittance allows for the use of in-situ analytical techniques, such as spectroscopy, to monitor reaction kinetics, identify intermediate species, and gain a deeper understanding of the process in real-time.
Understanding the Trade-offs
While its properties are exceptional, quartz is not the universal solution for every application. A clear understanding of its limitations is essential for making an informed decision.
The Brittleness Factor
Like other ceramics and glasses, quartz is strong under compression but is inherently brittle. It can easily fracture or shatter from sharp physical impacts.
Careful handling is required, as accidental drops or collisions can lead to catastrophic failure of the cell.
Cost and Fabrication
High-purity quartz is significantly more expensive than common laboratory materials like borosilicate glass (e.g., Pyrex).
Furthermore, its hardness makes it difficult and costly to machine or form into complex shapes, which can limit the design possibilities for custom electrolytic cells.
Making the Right Choice for Your Application
The decision to use quartz should be driven by the specific demands of your electrochemical process.
- If your primary focus is analytical precision and purity: Quartz is the superior choice due to its unmatched chemical inertness.
- If your primary focus is high-temperature or high-energy processes: The thermal and electrical properties of quartz are critical for safe and reliable operation.
- If your primary focus is real-time spectroscopic analysis: The broad optical transparency of quartz is a non-negotiable requirement.
- If your primary focus is general-purpose use on a budget: A material like borosilicate glass may be a more practical alternative, provided extreme conditions are not present.
Choosing the right material is the foundation for achieving accurate, repeatable, and safe electrochemical results.
Summary Table:
| Key Feature | Benefit in Electrolytic Cells |
|---|---|
| Chemical Stability | Resists corrosion from strong acids/bases, ensuring purity and preventing contamination. |
| High-Temperature Resistance | Withstands extreme heat and thermal shock, maintaining structural integrity. |
| Electrical Insulation | Prevents short-circuiting, directing all current through the electrolyte for efficient reactions. |
| Optical Transparency | Enables real-time, in-situ spectroscopic analysis (UV to IR) for process monitoring. |
Ready to enhance the precision and reliability of your electrochemical processes?
At KINTEK, we specialize in providing high-quality quartz lab equipment tailored to the demanding needs of modern laboratories. Our quartz electrolytic cells are engineered to deliver unmatched chemical inertness, thermal stability, and optical clarity, ensuring the integrity of your most critical experiments.
Whether you are developing new materials, conducting advanced analytical studies, or running high-temperature syntheses, KINTEK has the solution to support your success.
Contact our experts today to discuss your specific application and discover how our quartz products can empower your research.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What are the available volumes and dimensions for the all-quartz electrolytic cell? Find the Perfect Fit for Your Lab
- What are the necessary steps to prepare an all-quartz electrolytic cell before an experiment? Ensure Accuracy and Safety



















