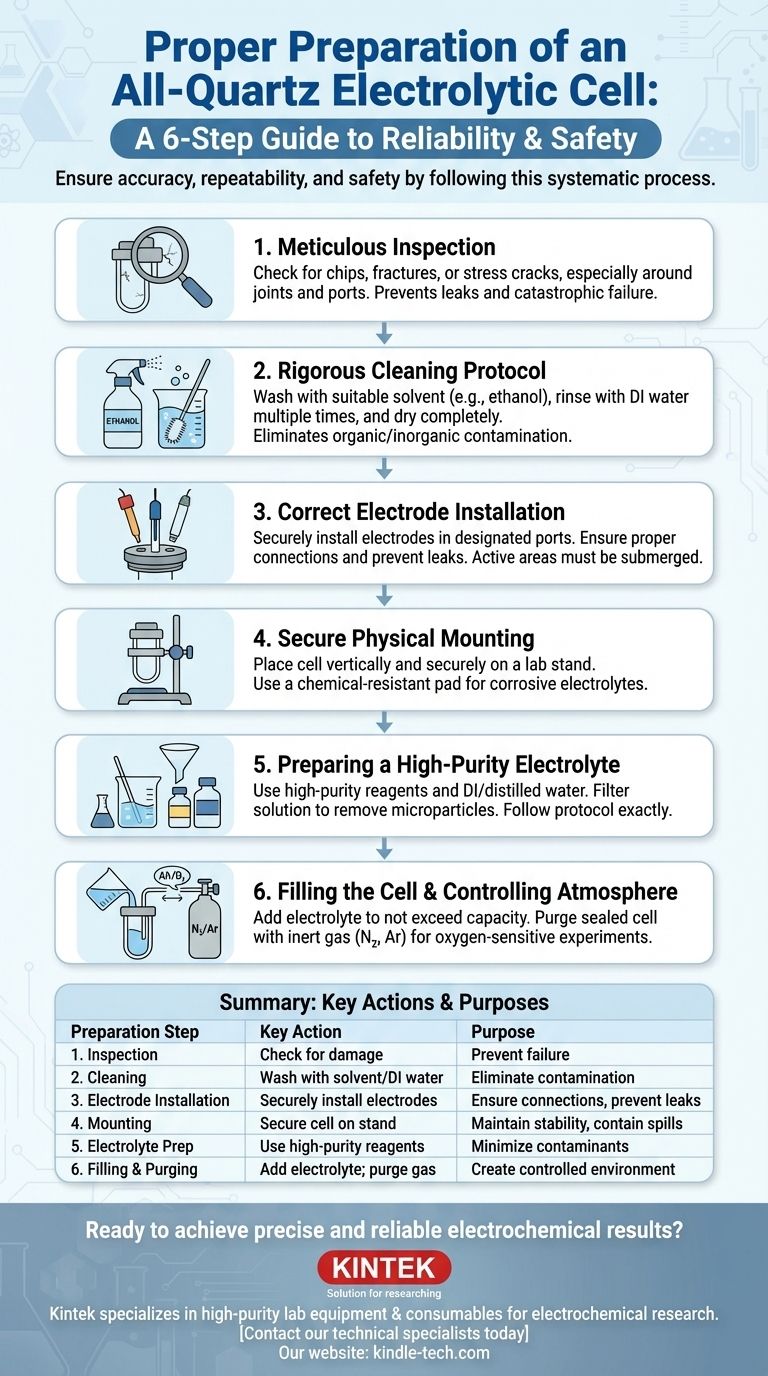
Proper preparation of an all-quartz electrolytic cell is a systematic process that ensures experimental accuracy, repeatability, and safety. The necessary steps involve inspecting the cell for damage, performing a rigorous cleaning procedure, correctly installing the electrodes, and carefully preparing and adding the electrolyte while controlling the internal atmosphere. Each step is critical for eliminating variables that could compromise your results.
The goal of preparation is not merely to assemble the equipment, but to create a perfectly controlled environment. Meticulous preparation is the foundation of reliable electrochemical data, as it minimizes contamination and ensures that the only reactions occurring are the ones you intend to study.

Foundational Steps: Cell Integrity and Cleanliness
Before any components are assembled, the vessel itself must be verified as sound and analytically clean. These initial steps prevent catastrophic failure and data contamination.
Step 1: Meticulous Inspection
Always begin by carefully examining the all-quartz cell. Check for any chips, fractures, or stress cracks, especially around the joints and electrode ports.
A compromised cell is not only a risk for leaks but can also fail under minor thermal or mechanical stress during the experiment, jeopardizing your work and your safety.
Step 2: Rigorous Cleaning Protocol
Thoroughly clean the cell to remove any organic or inorganic impurities. A common procedure involves washing with a suitable solvent, such as high-purity ethanol, followed by multiple rinses with deionized (DI) water.
The goal is to leave a surface that is free of any residue that could act as an unwanted catalyst, inhibitor, or redox species in your experiment. Ensure the cell is completely dry before assembly.
Assembling the Electrochemical System
With a clean and intact cell, the next phase is the physical assembly of the electrodes and the secure mounting of the apparatus.
Step 3: Correct Electrode Installation
Install the working, reference, and counter electrodes into their designated ports. Ensure the connections are tight to prevent leaks and maintain the desired atmosphere.
The active areas of the electrodes must be fully submerged in the electrolyte once filled. However, take care that the electrolyte level does not reach the upper connecting pins or rods, which could cause short circuits or corrosion.
Step 4: Secure Physical Mounting
Place the assembled electrolytic cell onto the base of a laboratory stand and tighten the fixing knobs. The cell should be perfectly vertical and stable.
If you are using a corrosive electrolyte, place a chemical-resistant, leak-proof pad underneath the cell as a secondary containment measure.
Establishing the Chemical Environment
The final preparation stage involves creating the precise chemical conditions required for your experiment inside the sealed cell.
Step 5: Preparing a High-Purity Electrolyte
Prepare your electrolyte solution using high-purity chemical reagents and either distilled or deionized water. Contaminants in the reagents or solvent are a primary source of experimental error.
Follow your experimental protocol exactly for all proportions and mixing methods. It is often wise to filter the final electrolyte solution to remove any suspended microparticles.
Step 6: Filling the Cell and Controlling Atmosphere
Pour the prepared electrolyte into the cell, ensuring the volume does not exceed its maximum rated capacity.
If your experiment is sensitive to oxygen or other atmospheric components, you must purge the cell. Before adding the electrolyte, flush the sealed cell with an inert gas, like high-purity nitrogen or argon, to remove the internal air.
Common Pitfalls and Safety Considerations
Mistakes during preparation are a common source of failed experiments. Being aware of these pitfalls and adhering to safety protocols is non-negotiable.
Contamination from Handling
Avoid touching the internal surfaces of the cell or the active surfaces of the electrodes with your bare hands. Oils and residues from your skin can easily contaminate the system.
Electrical and Chemical Hazards
Once the experiment begins, the system will be live. Avoid direct contact with the electrodes and electrolyte to prevent electric shock and chemical burns. Keep all flammable materials and open flames far away from the setup.
Overlooking Atmospheric Control
Many electrochemical reactions are highly sensitive to the presence of oxygen, which is electrochemically active. Failing to properly purge the cell with an inert gas is a frequent cause of unexpected or non-reproducible results.
Final Checks Before You Begin
Use these final points to confirm your setup aligns with your primary experimental goal.
- If your primary focus is data accuracy and repeatability: Confirm that your cleaning was meticulous, your reagents were high-purity, and you have established complete control over the cell's atmosphere.
- If your primary focus is experimental safety: Double-check the quartz cell for any cracks, ensure the apparatus is securely mounted, and review the chemical and electrical hazards before applying power.
- If your primary focus is a successful first run: Methodically follow the sequence—inspect, clean, assemble, fill, and purge—and verify that each electrode is properly connected and submerged.
By treating preparation as a critical phase of the experiment itself, you ensure the integrity and safety of your work from the very start.
Summary Table:
| Preparation Step | Key Action | Purpose |
|---|---|---|
| 1. Inspection | Check for chips, fractures, or stress cracks. | Prevent leaks and catastrophic failure. |
| 2. Cleaning | Wash with solvent (e.g., ethanol) and rinse with DI water. | Eliminate organic/inorganic contamination. |
| 3. Electrode Installation | Securely install working, reference, and counter electrodes. | Ensure proper connections and prevent leaks. |
| 4. Mounting | Secure cell vertically on a stand with a leak-proof pad. | Maintain stability and contain potential spills. |
| 5. Electrolyte Prep | Use high-purity reagents and DI/deionized water; filter if needed. | Minimize contaminants that cause experimental error. |
| 6. Filling & Purging | Add electrolyte; purge with inert gas (N₂, Ar) for oxygen-sensitive experiments. | Create a controlled chemical environment. |
Ready to achieve precise and reliable electrochemical results?
The meticulous preparation of your electrolytic cell is fundamental to your success. KINTEK specializes in high-purity lab equipment and consumables, including quartz cells and electrodes, designed to meet the exacting demands of electrochemical research.
Let our expertise support your work. Contact our technical specialists today to discuss your specific laboratory needs and ensure your experiments are built on a foundation of quality and reliability.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the standard opening specifications for sealed and unsealed all-quartz electrolytic cells? Optimize Your Electrochemistry Setup
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance



















