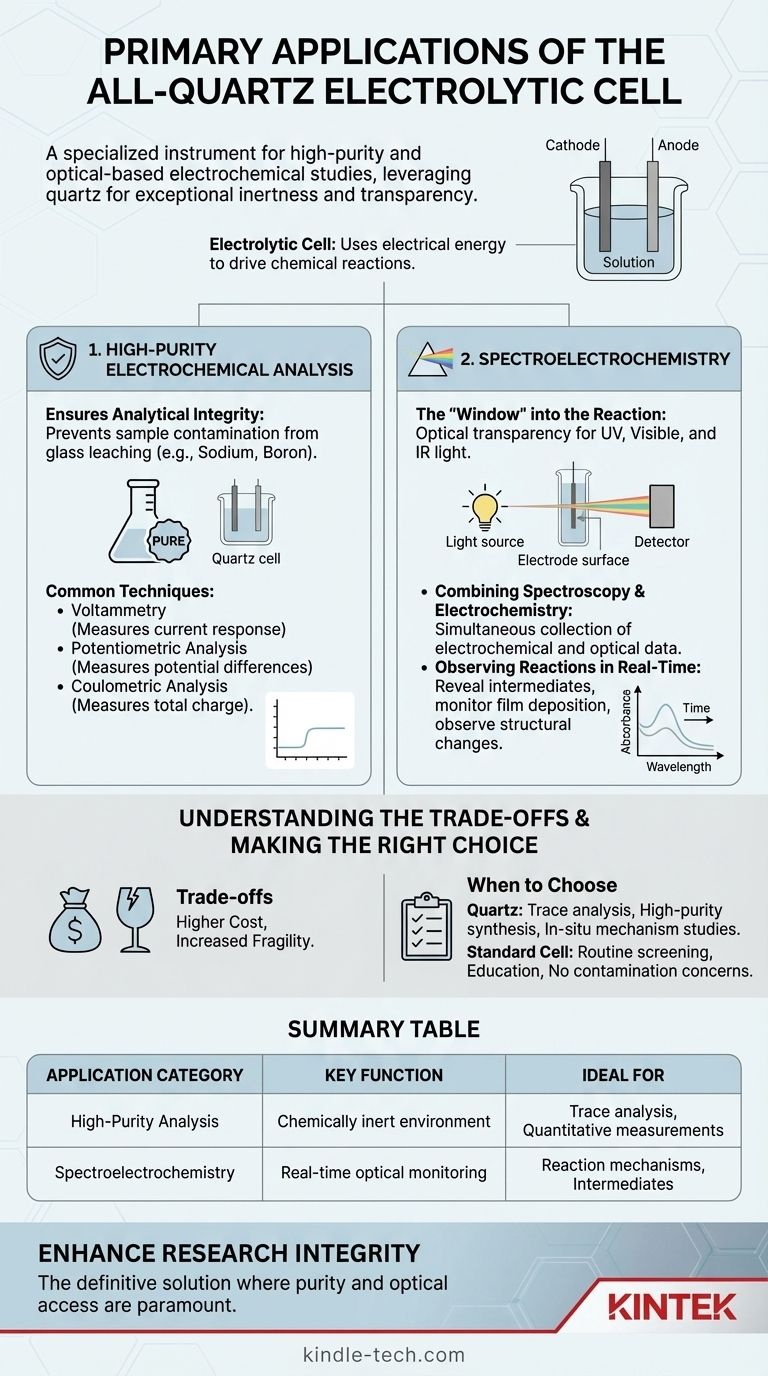
At its core, the all-quartz electrolytic cell is a specialized instrument designed for high-purity and optical-based electrochemical studies. Its primary applications fall into two main categories: high-precision electrochemical analysis where chemical purity is paramount, and spectroelectrochemistry, which leverages the cell's transparency to light. The choice of quartz is deliberate, providing exceptional chemical inertness and a clear window for optical measurements that standard glass cannot offer.
The decision to use an all-quartz cell is driven by two critical requirements: eliminating contamination from glass components and enabling in-situ optical measurements of reactions on an electrode. Its unique properties are essential for the most sensitive and demanding experiments.

The Role of an Electrolytic Cell in Research
What is an Electrolytic Cell?
An electrolytic cell is a fundamental tool in chemistry that uses an external source of electrical energy to drive a chemical reaction that would not occur on its own.
Unlike a battery which generates electricity from a spontaneous reaction, an electrolytic cell consumes electricity. This process involves a negative cathode and a positive anode, with ions moving through a solution to complete the circuit.
Why "All-Quartz"? The Material Advantage
Standard laboratory glassware is typically made of borosilicate glass. While robust, it can slowly leach ions, such as sodium or boron, into the solution.
In highly sensitive experiments, this leaching can act as a contaminant, altering reaction pathways or interfering with analytical measurements. Quartz, which is essentially pure silicon dioxide, is exceptionally chemically inert and does not have this problem, ensuring the purity of the experiment.
Primary Application 1: High-Purity Electrochemical Analysis
Ensuring Analytical Integrity
For techniques that measure minute changes in current or voltage, even trace impurities can skew results. The inert nature of an all-quartz cell provides a clean, stable, and predictable environment.
This guarantees that the observed electrochemical signals originate purely from the intended reaction, not from unintended side reactions with contaminants.
Common Techniques
The stability of an all-quartz cell makes it ideal for several precise analytical methods.
- Voltammetry: Measures the current response to a changing voltage. A clean system is critical for interpreting the resulting voltammogram accurately.
- Potentiometric Analysis: Measures potential differences under zero current conditions. Purity is essential for stable and reproducible reference potentials.
- Coulometric Analysis: Measures the total charge passed to determine the amount of substance reacted. Any side reaction caused by impurities leads to direct errors in quantification.
Primary Application 2: Spectroelectrochemistry
The "Window" into the Reaction
The second key property of quartz is its optical transparency across a wide range of the electromagnetic spectrum, including ultraviolet (UV), visible, and near-infrared (IR) light.
This transparency allows the cell to function as a window, enabling researchers to shine a beam of light through the solution and directly onto the electrode surface while an electrochemical experiment is running.
Combining Spectroscopy and Electrochemistry
This combined technique is known as spectroelectrochemistry. It allows for the simultaneous collection of electrochemical data (current/voltage) and spectroscopic data (how the substance absorbs or reflects light).
This provides a much richer understanding than either technique could alone, directly correlating electrical changes with changes in chemical structure or concentration.
Observing Reactions in Real-Time
Using spectroelectrochemistry, a scientist can watch a reaction as it happens.
This can reveal the formation of short-lived colored intermediates, monitor the deposition of a thin film onto an electrode, or observe changes in a molecule's structure as it is oxidized or reduced.
Understanding the Trade-offs
Cost and Fragility
Quartz is significantly more expensive to produce and machine than borosilicate glass. This makes all-quartz cells a considerable investment compared to standard cells.
Furthermore, while thermally resistant, quartz is more brittle and less resistant to mechanical shock than borosilicate glass, requiring more careful handling.
When a Simpler Cell is Sufficient
The specialized nature of an all-quartz cell means it is not necessary for every application.
For general-purpose electrochemistry, educational demonstrations, or experiments where trace contamination or optical access is not a concern, a standard glass or polymer cell is often a more practical and cost-effective solution.
Making the Right Choice for Your Experiment
Choosing the correct cell is a foundational decision based on your experimental goals.
- If your primary focus is trace analysis or high-purity synthesis: The chemical inertness of an all-quartz cell is essential to prevent sample contamination and ensure accurate results.
- If your primary focus is studying reaction mechanisms in-situ: The optical transparency of the quartz cell is non-negotiable for performing spectroelectrochemical analysis.
- If your primary focus is routine electrochemical screening or education: A standard borosilicate glass or polymer cell is almost always the more cost-effective and durable choice.
Ultimately, selecting the correct cell material is a critical step in ensuring the integrity and success of your electrochemical research.
Summary Table:
| Application Category | Key Function | Ideal For |
|---|---|---|
| High-Purity Electrochemical Analysis | Provides a chemically inert environment to prevent contamination. | Trace analysis, voltammetry, coulometry, and sensitive quantitative measurements. |
| Spectroelectrochemistry | Enables real-time optical monitoring of reactions through UV-Vis-IR transparent quartz. | Studying reaction mechanisms, identifying intermediates, and correlating structural changes with electrical data. |
Enhance the Integrity of Your Electrochemical Research
Choosing the right equipment is fundamental to achieving accurate, contamination-free results. The all-quartz electrolytic cell is the definitive solution for experiments where purity and optical access are paramount.
KINTEK specializes in high-precision lab equipment and consumables, serving the exacting needs of research and quality control laboratories. Our expertise ensures you have the right tools for your most demanding electrochemical studies.
Ready to achieve superior analytical integrity?
Contact our experts today to discuss how our all-quartz electrolytic cells can advance your research.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How should an all-quartz electrolytic cell and its components be maintained for long-term use? A Guide to Maximizing Equipment Lifespan
- What is the proper procedure for post-experiment cleanup and storage of an all-quartz electrolytic cell? Ensure Longevity and Reproducibility
- What materials are used to construct the all-quartz electrolytic cell? A Guide to Purity and Performance
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab



















