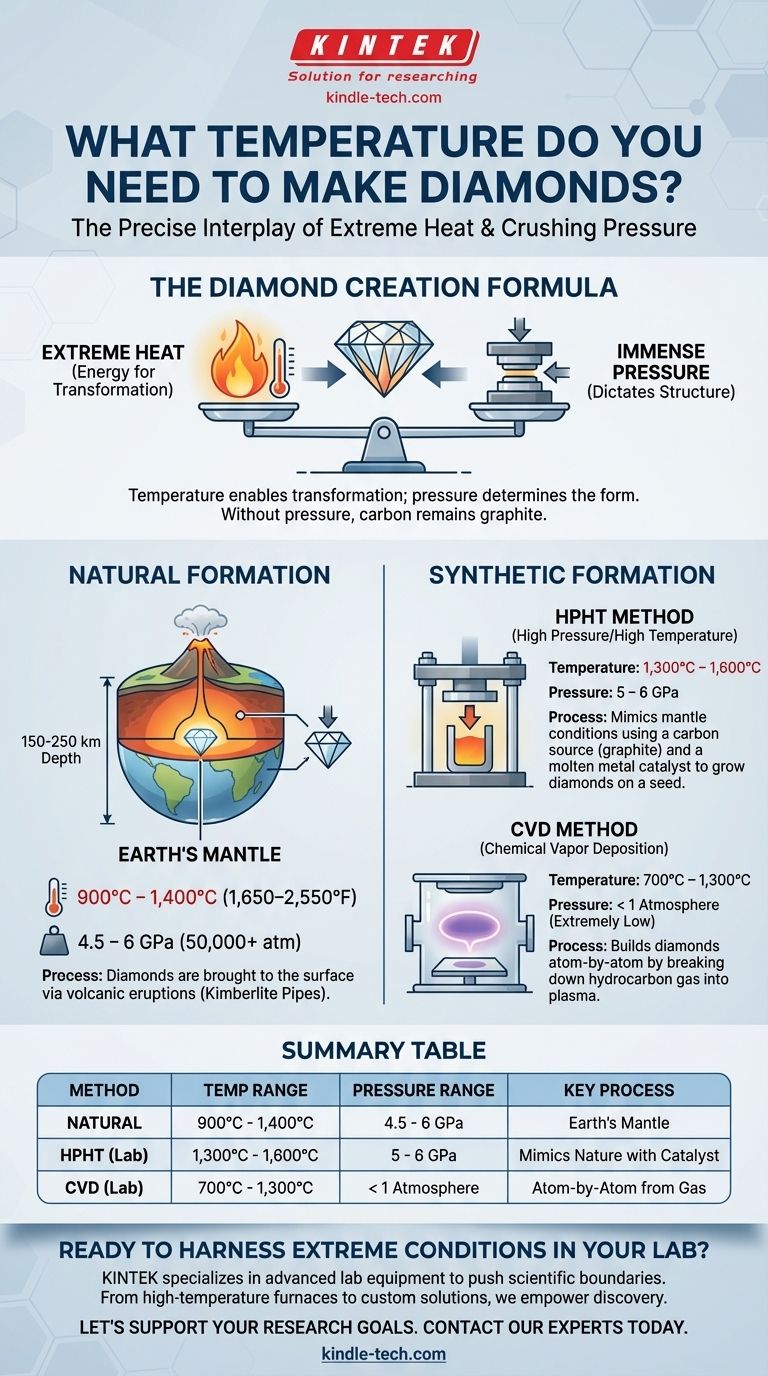
To create a diamond, you need extremely high temperatures, but the exact number depends entirely on the pressure you can apply. In nature, diamonds form at temperatures between 900°C and 1,400°C (1,650–2,550°F), while the most common lab-grown method, HPHT, uses similar temperatures of around 1,300–1,600°C. However, temperature is only half of the equation; without immense pressure, you will only ever make graphite.
The creation of a diamond is not a function of temperature alone, but a precise interplay between extreme heat and crushing pressure. Understanding this relationship is the key to understanding how carbon can transform from its common form, graphite, into one of the hardest and most valued materials on Earth.

The Two Paths to Diamond Creation
Diamonds are simply carbon atoms arranged in a specific, highly dense crystal structure. To force those atoms into this formation, nature and science use two primary methods, each with a distinct recipe of heat and pressure.
Natural Formation: The Earth's Mantle
Natural diamonds form deep within the Earth's upper mantle, roughly 150 to 250 kilometers below the surface.
At these depths, the necessary conditions are met:
- Temperature: Approximately 900°C to 1,400°C (1,650–2,550°F).
- Pressure: An immense 4.5 to 6 gigapascals (GPa). This is over 50,000 times the atmospheric pressure at sea level.
These diamonds are then carried to the surface over millions of years through deep-source volcanic eruptions, which create the kimberlite pipes where most diamonds are mined today.
Synthetic Formation: The Laboratory
Scientists have developed two main techniques to replicate and even innovate upon nature's process.
The HPHT Method (High Pressure/High Temperature)
This method most closely mimics the conditions in the Earth's mantle. A carbon source, like graphite, is placed in a large mechanical press.
- Temperature: A crucible heats the carbon to 1,300°C–1,600°C.
- Pressure: The press applies pressures of 5 to 6 GPa.
A molten metal catalyst is used to dissolve the carbon, which then crystallizes around a tiny diamond "seed" to form a larger, gem-quality diamond.
The CVD Method (Chemical Vapor Deposition)
CVD takes a completely different approach, building a diamond atom by atom. It's less about brute force and more about precise chemical control.
- Temperature: A hydrocarbon gas (like methane) is heated in a vacuum chamber to 700°C–1,300°C.
- Pressure: The pressure is extremely low, often below one atmosphere.
The heat breaks the gas down into a plasma of carbon ions, which then deposit onto a flat diamond seed plate, growing a diamond layer by layer.
Understanding the Trade-offs: Why Pressure Is the Decisive Factor
Many people wonder why you can't just heat carbon to make a diamond. The answer lies in the carbon phase diagram, which maps the stable form of carbon at different temperatures and pressures.
Graphite: The Default State
At the pressures we experience in daily life (one atmosphere), carbon's most stable form is graphite.
Even if you heat graphite to 3,000°C, it will remain graphite or sublimate into a gas. It simply does not have the external force needed to compel its atoms into the tightly packed diamond structure.
Diamond: The High-Pressure State
Applying immense pressure is what changes the rules. Pressure physically forces the carbon atoms closer together, making the denser diamond structure more stable than the less-dense graphite structure.
Temperature's role is to provide energy. It gives the carbon atoms the mobility they need to break their existing bonds and rearrange into the new, stable diamond lattice once the pressure is applied. Without sufficient heat, the process would take an impossibly long time, even at the correct pressure.
Making the Right Choice for Your Goal
Your interest in the temperature required to make diamonds likely stems from a deeper curiosity about the process itself. Understanding your goal will clarify which process is more relevant to you.
- If your primary focus is on geology and natural wonders: You should focus on the conditions of the Earth's mantle—temperatures around 1,000°C combined with pressures exceeding 5 GPa.
- If your primary focus is on industrial manufacturing and technology: The HPHT method is the most direct mimic of nature, while the CVD method represents a more advanced, controlled approach that allows for different applications.
- If your primary focus is on the core scientific principle: The key is that temperature enables transformation, but pressure dictates what that transformation will be.
Ultimately, turning simple carbon into a diamond is a powerful demonstration of how physical conditions define the structure of matter.
Summary Table:
| Method | Temperature Range | Pressure Range | Key Process |
|---|---|---|---|
| Natural Formation | 900°C - 1,400°C | 4.5 - 6 GPa | Forms in Earth's mantle |
| HPHT (Lab-Grown) | 1,300°C - 1,600°C | 5 - 6 GPa | Mimics natural conditions with a catalyst |
| CVD (Lab-Grown) | 700°C - 1,300°C | < 1 Atmosphere | Builds diamond atom-by-atom from gas |
Ready to Harness Extreme Conditions in Your Lab?
Understanding the precise interplay of heat and pressure is fundamental to materials science. Whether your research involves high-temperature synthesis, material testing, or developing new carbon-based materials, having the right equipment is critical.
KINTEK specializes in the advanced lab equipment you need to push the boundaries of science. From high-temperature furnaces capable of reaching over 1,600°C to custom solutions for controlled environments, we provide the tools that empower discovery and innovation in laboratories worldwide.
Let's discuss how we can support your specific research goals. Contact our experts today to find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- CVD Diamond for Thermal Management Applications
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- How hot isostatic pressing works? Achieve 100% Density in Your High-Performance Components
- What function does the pressure applied by a vacuum hot press furnace serve? Enhance Ti-Al3Ti Composite Sintering
- What is the role of SPS equipment in Ti-Nb-Zr-O alloy fabrication? Achieve Rapid Densification & Precise Microstructure
- What are the functions of hydraulic pressure in diffusion bonding? Master Superior Composite Material Integration
- What is the effect of increasing the pressure during sintering? Achieve Maximum Density and Superior Performance
- What are the technical advantages of vacuum hot pressing? Optimize SiCp/6061 Composite Performance
- What are the advantages of using Vacuum Hot Pressing (VHP) equipment? Achieve Superior ODS Steel Density & Structure
- What are the process advantages of using a vacuum hot press vs. HIP? Simplify your steel preparation workflow.



















