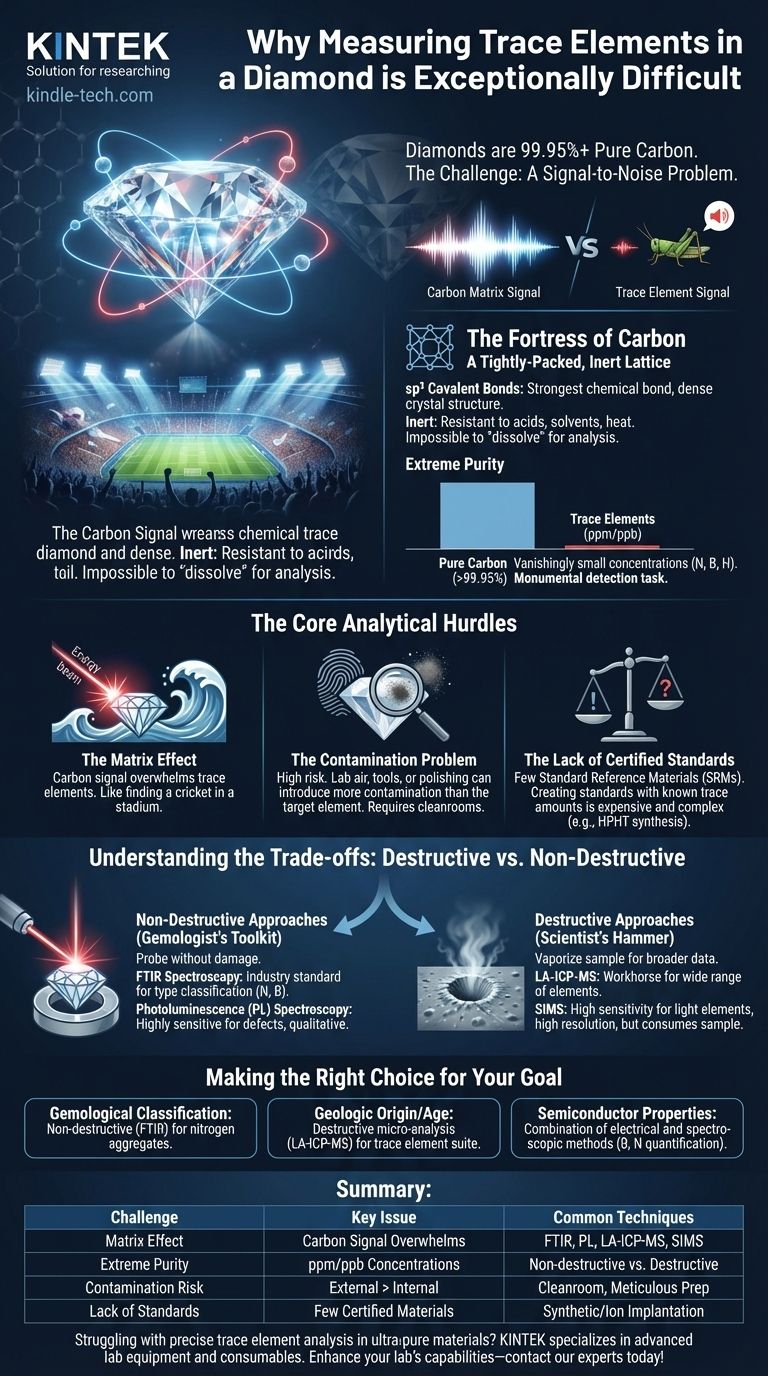
Measuring trace elements in a diamond is exceptionally difficult because of the material's unparalleled purity and the immense strength of its atomic structure. The carbon atoms in a diamond are locked in a dense, covalent lattice that is chemically inert and physically robust. This creates a formidable analytical challenge, requiring highly specialized techniques to detect the vanishingly small concentrations of foreign elements without destroying the sample or introducing contamination.
At its core, the difficulty is a signal-to-noise problem. The overwhelming signal from the carbon matrix masks the minuscule signals from trace elements, while the diamond's inertness makes it nearly impossible to prepare for analysis without introducing more contamination than you are trying to measure.

The Fortress of Carbon: Why the Diamond Matrix Resists Analysis
To understand the difficulty, you must first appreciate the unique nature of the diamond itself. It is not just a hard mineral; it is a near-perfect crystalline structure.
A Tightly-Packed, Inert Lattice
A diamond is composed of carbon atoms linked by sp³ covalent bonds, the strongest type of chemical bond. This creates an incredibly dense and stable crystal lattice.
This structure is highly resistant to acids, solvents, and heat. You cannot simply "dissolve" a diamond to release its trace elements for analysis, a common first step for many other materials.
Extreme Purity by Nature
Diamonds form under immense pressure and heat deep within the Earth's mantle. This environment is a natural purification process, resulting in a material that is often more than 99.95% pure carbon.
Trace elements like nitrogen, boron, or hydrogen are present in concentrations measured in parts per million (ppm) or even parts per billion (ppb). Detecting such a tiny minority of atoms within a vast majority of carbon atoms is a monumental task.
The Core Analytical Hurdles
Scientists face several fundamental obstacles when attempting to quantify what little is "not carbon" inside a diamond.
The "Matrix Effect": Drowning in Carbon
Most analytical instruments work by bombarding a sample with energy (like lasers or ion beams) and measuring what is emitted. In a diamond, nearly all the energy interacts with carbon atoms.
This creates a massive "matrix signal" from carbon that can easily overwhelm the faint, almost imperceptible signal from a trace element. It’s like trying to hear a single cricket chirping in the middle of a roaring stadium.
The Contamination Problem
Because diamonds are so pure, the risk of contamination is extremely high. A single fingerprint, a speck of dust, or even the air in the lab can contain higher concentrations of certain elements than the diamond itself.
Preparing a diamond for analysis—such as polishing a surface or cleaning it—can inadvertently introduce more analytical "noise" than the signal you are trying to find. This requires cleanroom conditions and meticulous handling procedures.
The Lack of Certified Standards
To get an accurate quantitative measurement (e.g., "this diamond contains 10 ppm of boron"), you must first calibrate your instrument using a standard reference material (SRM). An SRM is a material with a precisely known concentration of the element you're measuring.
Creating a diamond standard is exceptionally difficult. It involves complex processes like ion implantation or high-pressure/high-temperature (HPHT) synthesis to produce a diamond with a known amount of a trace element, a process that is both expensive and technically demanding.
Understanding the Trade-offs: Destructive vs. Non-Destructive Methods
No single technique can answer all questions about a diamond's composition. The choice of method always involves a critical trade-off, primarily between obtaining detailed data and preserving the sample.
Non-Destructive Approaches (The Gemologist's Toolkit)
For valuable gemstones, non-destructive analysis is essential. These methods probe the diamond without causing any damage.
Fourier Transform Infrared (FTIR) Spectroscopy is the industry standard for classifying diamond types. It excels at detecting and quantifying nitrogen and boron when they are present in sufficient concentrations, as these elements absorb specific frequencies of infrared light.
Photoluminescence (PL) Spectroscopy uses a laser to cause specific atomic defects (often involving trace elements) to glow. It is incredibly sensitive for detecting certain elements but is not a bulk analysis technique and is difficult to use for precise quantification.
Destructive Approaches (The Scientist's Hammer)
To get a broader and more sensitive elemental "fingerprint," geoscientists often must resort to methods that damage the sample, typically by vaporizing a microscopic amount of it.
Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS) is a workhorse technique. A laser blasts a microscopic crater in the diamond, and the resulting vapor is sent to a mass spectrometer that can measure a wide range of trace elements.
Secondary Ion Mass Spectrometry (SIMS) offers even greater sensitivity for light elements like nitrogen and hydrogen. It uses a focused ion beam to sputter atoms from the diamond's surface, providing high-resolution data but at the cost of sample destruction.
Making the Right Choice for Your Goal
The "best" analytical approach depends entirely on the question you need to answer.
- If your primary focus is gemological classification (e.g., Type Ia vs. IIa): Rely on non-destructive FTIR analysis, as it is the standard for quantifying the nitrogen aggregates that define a diamond's type.
- If your primary focus is determining geologic origin or age: You will likely need destructive micro-analysis like LA-ICP-MS to measure a wide suite of trace elements trapped in the diamond or its mineral inclusions.
- If your primary focus is studying semiconductor properties for electronics: Use a combination of electrical measurements and spectroscopic techniques to quantify the concentration and state of boron or nitrogen, which control the diamond's electronic behavior.
Ultimately, analyzing a diamond requires choosing the right tool for a specific question, always balancing the need for precise data with the preservation of the unique and valuable sample.
Summary Table:
| Challenge | Key Issue | Common Analytical Techniques |
|---|---|---|
| Matrix Effect | Carbon signal overwhelms trace element signals | FTIR, PL Spectroscopy, LA-ICP-MS, SIMS |
| Extreme Purity | Trace elements present in ppm/ppb concentrations | Non-destructive (FTIR, PL) vs. Destructive (LA-ICP-MS, SIMS) |
| Contamination Risk | External elements can exceed internal traces | Cleanroom handling, meticulous sample preparation |
| Lack of Standards | Few certified reference materials available | Reliance on synthetic diamonds or ion implantation for calibration |
Struggling with precise trace element analysis in ultra-pure materials like diamonds? KINTEK specializes in advanced lab equipment and consumables tailored for challenging analytical tasks. Our solutions help you overcome matrix effects, contamination, and sensitivity limits—ensuring accurate results for gemology, geology, and materials science. Enhance your lab's capabilities—contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Filter Testing Machine FPV for Dispersion Properties of Polymers and Pigments
People Also Ask
- Why is a precision vibrating sieve shaker essential for metal leaching research? Optimize Your Particle Size Analysis
- What is the role of standard sieves in gold scrap leaching kinetic studies? Ensure Precision in Particle Classification
- What is the primary purpose of using standard sieves? Master Particle Uniformity for High-Quality Catalyst Preparation
- What is the function of sieving equipment in CuAlMn alloys? Master Pore Size Precision
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
















