In short, Potassium Bromide (KBr) is inactive in infrared (IR) spectroscopy because its crystal lattice vibrations do not cause a change in its overall dipole moment. Since absorbing IR radiation is fundamentally dependent on a molecule's dipole moment changing as it vibrates, KBr does not absorb the radiation and is therefore transparent in the mid-IR range.
The inactivity of KBr is not a flaw; it is a critical feature. Materials like KBr are deliberately chosen for IR analysis because they provide a transparent "window," allowing the spectrometer to measure the vibrations of the sample alone without interference.

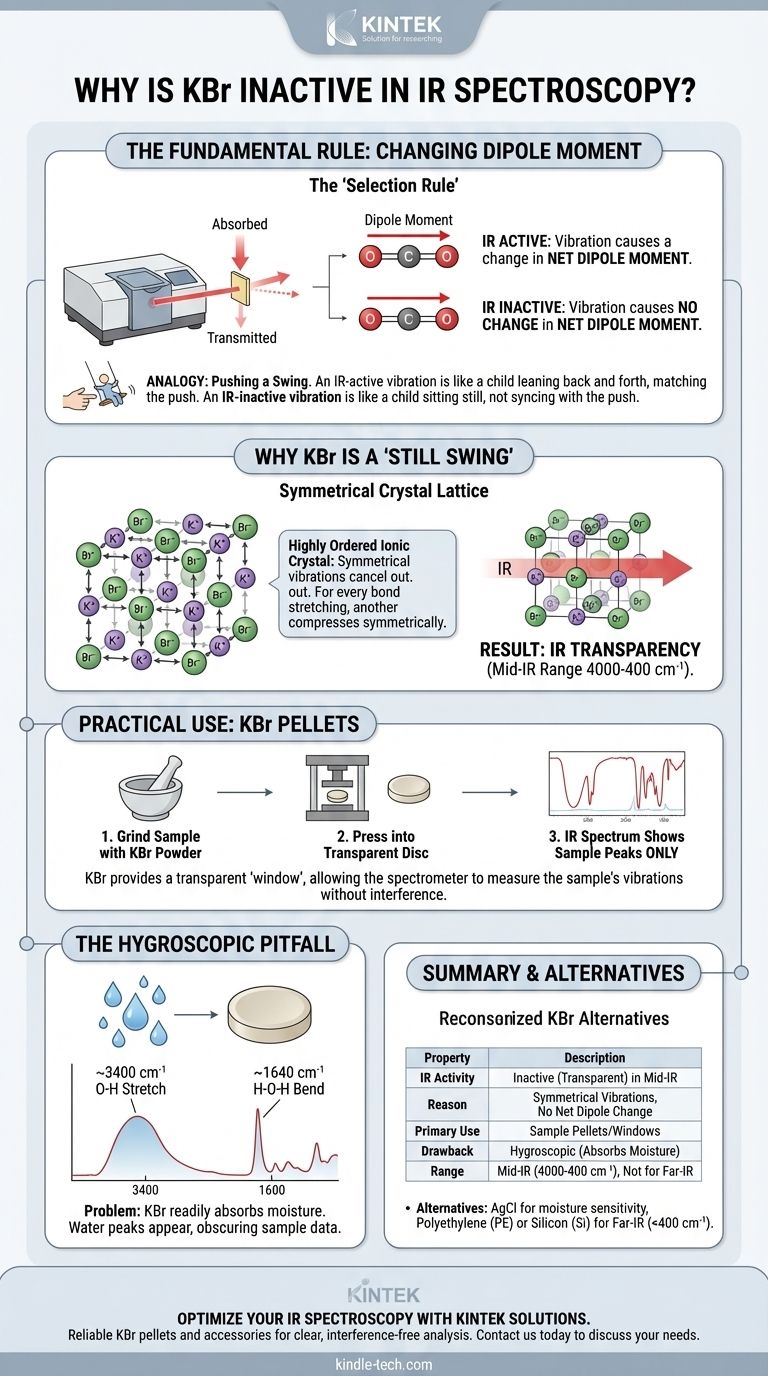
The Fundamental Rule of IR Spectroscopy
To understand why KBr is inactive, we must first understand the single most important requirement for a molecule to be IR active.
The "Changing Dipole Moment" Requirement
IR spectroscopy works by shining infrared light on a sample and measuring which frequencies of light are absorbed.
A molecule only absorbs IR radiation at a specific frequency if that radiation matches the frequency of one of its natural vibrations (like stretching or bending).
Crucially, for the energy to be transferred, the vibration must cause a change in the molecule's net dipole moment. This is the absolute, non-negotiable "selection rule" of IR spectroscopy.
An Analogy: Pushing a Swing
Think of the oscillating electric field of the IR light as a hand trying to push a child on a swing.
An IR-active vibration (like the C=O stretch in acetone) is like a child leaning back and forth, changing their center of mass. The hand can time its pushes to match this movement and transfer energy, making the swing go higher.
An IR-inactive vibration is like a child sitting perfectly still on the swing. No matter how the hand tries to push, it can't transfer energy effectively. The vibration and the light are "out of sync."
Why KBr Is a "Still Swing"
KBr is an ionic compound, forming a highly ordered, symmetrical crystal lattice of K⁺ and Br⁻ ions. While the K-Br bond itself is extremely polar, its behavior within the solid crystal is what matters.
Symmetrical Vibrations in a Crystal
In the solid KBr lattice, the ions can vibrate. The primary vibration is a "stretching" motion between adjacent K⁺ and Br⁻ ions.
However, because the crystal is so uniform and symmetrical, for every bond that stretches, a neighboring bond is also stretching or compressing in a way that cancels out any potential change in the overall electric field. The net dipole moment of the macroscopic crystal does not change.
The Result: IR Transparency
Since there is no oscillating dipole moment, the KBr crystal cannot absorb energy from the infrared light beam.
The light simply passes through the material unaffected, making KBr IR transparent across the most commonly used region of the spectrum (typically 4000 to 400 cm⁻¹).
Understanding the Trade-offs and Practical Use
This transparency makes KBr an exceptionally useful—but not perfect—tool for sample preparation in IR spectroscopy, most often as pellets or windows.
The KBr Pellet Method
For solid samples, a common technique is to grind a small amount of the sample with pure, dry KBr powder. This mixture is then pressed under high pressure to form a small, transparent disc or "pellet."
Because the KBr matrix is transparent, any absorption peaks seen in the resulting spectrum are due only to the analyte, not the KBr holding it.
The Hygroscopic Problem: A Major Pitfall
The single biggest drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
This is a frequent source of frustration in the lab. If the KBr is not kept perfectly dry, water will appear in your spectrum, potentially obscuring important peaks from your actual sample.
Recognizing Water Contamination
Water contamination in a KBr pellet is easy to spot. It produces two characteristic signals:
- A very broad, strong peak around 3400 cm⁻¹ (from the O-H stretching vibrations).
- A smaller, sharp peak around 1640 cm⁻¹ (from the H-O-H bending vibration).
The Far-IR Cutoff
While transparent in the mid-IR, KBr itself does begin to absorb light at very low frequencies. Its useful transmission range ends around 400 cm⁻¹, making it unsuitable for far-IR spectroscopy.
Making the Right Choice for Your Analysis
Understanding KBr's properties allows you to use it effectively and know when to choose an alternative.
- If your primary focus is routine mid-IR analysis of a stable solid: KBr is the industry standard and most cost-effective choice, but you must ensure it is properly dried.
- If your sample has critical peaks near 3400 or 1640 cm⁻¹: You must either take extreme measures to keep your KBr dry or use a non-hygroscopic alternative matrix like Silver Chloride (AgCl).
- If you are working in the far-IR region (below 400 cm⁻¹): You cannot use KBr. You must select a material specifically suited for that range, such as polyethylene (PE) or silicon (Si).
Ultimately, selecting the right sampling material is as critical as running the spectrometer itself.
Summary Table:
| Property | Description |
|---|---|
| IR Activity | Inactive (transparent) in mid-IR range (4000-400 cm⁻¹) |
| Reason | Symmetrical crystal lattice vibrations cause no net dipole moment change |
| Primary Use | Sample preparation as pellets or windows for IR spectroscopy |
| Key Advantage | Provides a transparent matrix to analyze sample vibrations alone |
| Main Drawback | Hygroscopic (absorbs moisture, leading to water peaks in spectrum) |
| Transmission Range | Mid-IR (4000-400 cm⁻¹), not suitable for far-IR (<400 cm⁻¹) |
Optimize Your IR Spectroscopy with KINTEK
Are you looking to achieve clear, interference-free IR spectra for your laboratory analysis? KINTEK specializes in providing high-quality laboratory equipment and consumables, including reliable KBr pellets and accessories designed for precise IR spectroscopy.
Our products help you:
- Prepare perfect KBr pellets with minimal moisture contamination
- Obtain accurate spectral data without matrix interference
- Enhance your lab's efficiency with dependable sampling materials
Let our expertise support your research and analytical needs. Contact us today to discuss how KINTEK's lab solutions can improve your spectroscopy results!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What are the components of hydraulic machines? Master the Core System for Maximum Power
- Do hydraulics need lubrication? Why Hydraulic Fluid is the Multi-Tasking Lifeblood of Your System
- How does the high-pressure, high-temperature (HPHT) method for diamond synthesis work? Master the Science of Gem Growth
- What steel is used for a hydraulic press? Choosing the Right Materials for High-Stress Performance
- How does a laboratory hydraulic press contribute to MIC testing? Ensure Precision in Stainless Steel Specimens
- What are the hazards of hydraulic machines? Understanding High-Pressure Fluid Injection and Mechanical Risks
- Who uses a hydraulic press? A Guide to Industries and Applications Requiring Massive Force
- What is a manual press? A Guide to Human-Powered Force and Precision



















