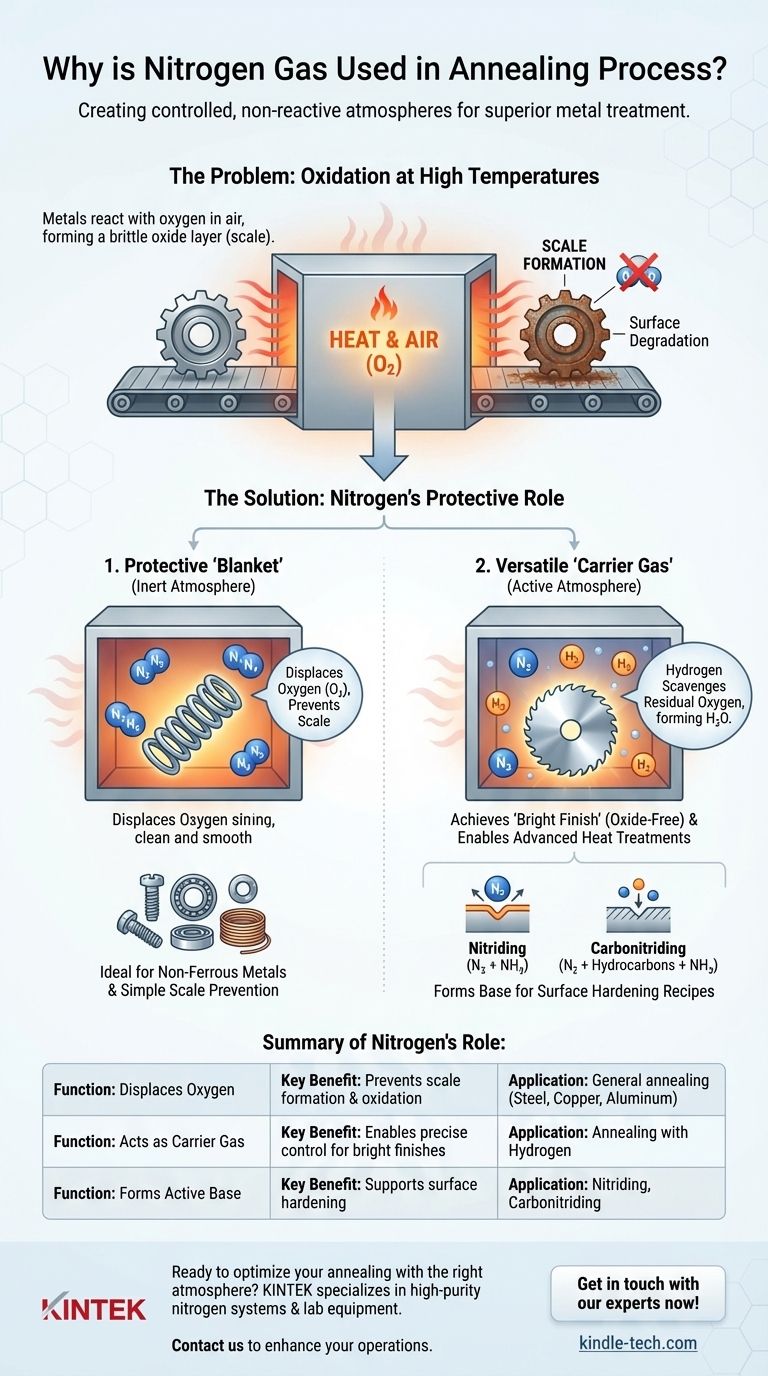
In short, nitrogen gas is used in the annealing process to create a controlled, non-reactive atmosphere that protects the metal from oxidation. By displacing the oxygen present in the air, nitrogen prevents the formation of scale and preserves the metal's surface integrity at the high temperatures required for annealing.
The core principle to understand is that nitrogen is more than just a passive "blanket." It serves as a versatile and cost-effective carrier gas, forming the base for precisely engineered atmospheres that can range from purely protective to chemically active, depending on the specific metallurgical goal.

The Fundamental Problem: Oxidation at High Temperatures
What is Annealing?
Annealing is a heat treatment process that alters a material's microstructure to achieve specific properties. It involves heating the metal to a designated temperature, holding it there, and then cooling it at a controlled rate.
This process is primarily used to relieve internal stresses, increase softness and ductility, and improve machinability.
The Problem with Air
When metals are heated to high temperatures, they react readily with the oxygen in the ambient air. This chemical reaction, known as oxidation, forms a brittle layer of metallic oxide, or "scale," on the surface.
This scale is undesirable as it degrades the surface finish, can interfere with subsequent processing steps, and alters the component's final dimensions.
Nitrogen's Role as a Protective Atmosphere
Displacing Oxygen: The Primary Function
The fundamental purpose of using nitrogen is to create an inert atmosphere inside the annealing furnace. Because nitrogen gas is largely non-reactive with most metals, it can safely displace the oxygen without causing its own adverse chemical reactions.
This protective atmosphere is crucial for a wide range of components, including screws, springs, bearings, saw blades, and non-ferrous metals like copper, aluminum, and brass.
Why Nitrogen?
Nitrogen is the gas of choice for this application due to its ideal combination of properties: it is effective, abundant (making up ~78% of Earth's atmosphere), and relatively low-cost to produce in high-purity forms.
Understanding the Trade-offs: Why Pure Nitrogen Isn't Always Enough
The Inevitability of Leaks
Industrial furnaces are not perfectly sealed systems. It is practically impossible to prevent small amounts of air (and thus oxygen) from leaking into the furnace chamber during operation.
The Limitation of Inertness
While nitrogen is excellent at displacing the bulk of the oxygen, it is chemically inert. This means it will not react with and neutralize the small amounts of oxygen that inevitably leak in.
For applications requiring a perfectly clean, oxide-free surface, even this minor amount of residual oxygen can be enough to cause slight discoloration or tarnishing.
Nitrogen as a Carrier Gas: The Key to Precision Control
Creating a Reducing Atmosphere for a 'Bright' Finish
To overcome the limitation of pure nitrogen, it is often used as a carrier gas for small percentages of an "active" or reducing gas, such as hydrogen.
This hydrogen actively seeks out and reacts with any free oxygen molecules, converting them to water vapor (H₂O). This process scavenges the residual oxygen, ensuring a truly oxygen-free environment and resulting in a pristine, mirror-like surface known as a bright finish.
Use in Other Heat Treatments
This same principle applies to other advanced heat treatments. Nitrogen acts as a carrier for specific reactive gases to intentionally modify the metal's surface.
- In nitriding, nitrogen carries ammonia to introduce nitrogen atoms into the steel's surface for hardening.
- In carbonitriding, nitrogen carries both hydrocarbon gases and ammonia to introduce carbon and nitrogen.
In all these cases, the nitrogen base provides a stable, controlled medium, while precisely metered additions of other gases perform the desired chemical work according to a specific "recipe."
Making the Right Choice for Your Goal
Achieving the desired outcome from annealing requires selecting the correct atmospheric composition for your specific objective.
- If your primary focus is simple scale prevention on non-critical parts: A high-purity nitrogen atmosphere is often sufficient and cost-effective.
- If your primary focus is a pristine, oxide-free 'bright' surface: You must use nitrogen as a carrier gas blended with a reducing agent like hydrogen.
- If your primary focus is surface hardening: You will use a nitrogen-based atmosphere that also carries specific reactive gases required for nitriding or carburizing.
Understanding nitrogen's role as a versatile carrier gas empowers you to select and control the precise atmosphere required for optimal heat treatment results.
Summary Table:
| Function | Key Benefit | Application |
|---|---|---|
| Displaces Oxygen | Prevents scale formation and oxidation | General annealing of steel, copper, aluminum |
| Acts as a Carrier Gas | Enables precise atmosphere control for bright finishes | Annealing with hydrogen for a mirror-like surface |
| Forms Base for Active Atmospheres | Supports surface hardening processes like nitriding | Advanced heat treatments requiring specific gas mixtures |
Ready to optimize your annealing process with the right atmosphere?
At KINTEK, we specialize in providing high-purity nitrogen systems and laboratory equipment tailored to your heat treatment needs. Whether you require simple scale prevention or advanced bright annealing capabilities, our solutions ensure precise atmospheric control for superior results.
Contact us today to discuss how our expertise in lab equipment and consumables can enhance your annealing operations and deliver the metal properties you need.
Get in touch with our experts now!
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vertical Laboratory Tube Furnace
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What are the principal methods for controlling the carbon potential of a generated furnace atmosphere? Master Precise Heat Treatment
- Why use a precision atmospheric control furnace for annealing HEAs? Unlock Pure Material Stability Data
- How does an Atmosphere Tube Furnace treat Na3SbS4-xSex electrolytes? Unlock High Ionic Conductivity
- Why is a high-purity hydrogen atmosphere furnace required for W-TiC pre-sintering? Achieve Pure Material Densification
- What are the common methods for producing protective gas atmospheres? Expert Heat Treating Solutions
- Why is a precisely controlled high-temperature furnace with steam or air atmospheres required? Engineering Alpha-Alumina
- What is hydrogen annealing? Achieve Superior Material Properties with Bright Annealing
- Why is controlled atmosphere important? Mastering Preservation and Industrial Processes



















