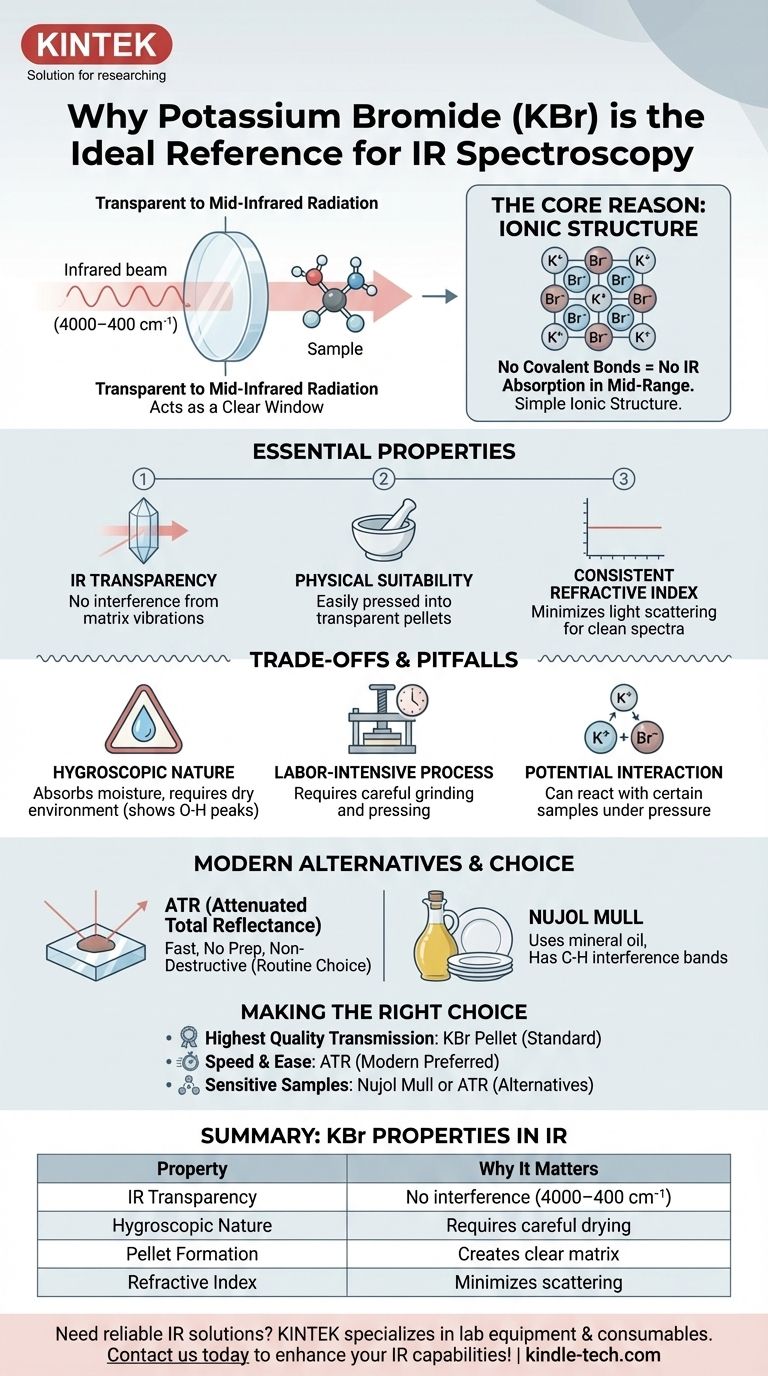
In short, potassium bromide (KBr) is used as a reference compound and sample matrix in IR spectroscopy because it is transparent to infrared radiation. Its simple ionic structure does not have molecular vibrations that absorb energy in the typical mid-infrared range (4000–400 cm⁻¹). This unique property allows it to act as a clear "window," holding the sample of interest so the spectrometer can measure the sample's spectrum without interference.
The fundamental reason for using KBr is its lack of covalent bonds, which means it has no molecular vibrations that absorb light in the mid-infrared range. This optical transparency allows the spectrometer to measure the sample's spectrum without interference from the surrounding material.

The Essential Properties of an IR Matrix
To understand why KBr is the traditional choice, it's necessary to understand what makes any material suitable for holding a sample for IR analysis. The ideal material must not interfere with the measurement itself.
The Principle of IR Transparency
Infrared spectroscopy works by measuring the absorption of IR light by the covalent bonds within a molecule, which causes them to vibrate (stretch, bend, etc.).
KBr is an ionic salt (K⁺Br⁻). It contains no covalent bonds. Because of this, it has no molecular vibrations that can be excited by mid-infrared radiation, making it effectively invisible to the spectrometer in the most useful analytical region.
Physical Suitability for Sample Preparation
KBr is a relatively soft, crystalline salt. When ground into a fine powder and subjected to high pressure (several tons), the crystals deform and fuse together.
This process creates a thin, semi-transparent or transparent solid disc, often called a KBr pellet. The sample, which was ground together with the KBr, becomes trapped within this solid, salt-based matrix, making it ideal for analysis.
A Consistent Refractive Index
A well-prepared KBr pellet has a uniform refractive index that helps minimize the scattering of infrared light. This reduction in scattering leads to a flatter baseline and a cleaner, more interpretable spectrum.
Understanding the Trade-offs and Common Pitfalls
While KBr is a traditional standard, it is not without its challenges. Awareness of these limitations is critical for generating high-quality data.
The Problem of Water Absorption
The most significant drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
Water (H₂O) has very strong, broad IR absorption bands, particularly the O-H stretching vibration around 3400 cm⁻¹. If your KBr is not kept perfectly dry, these water peaks can obscure important features of your sample's spectrum.
The Labor-Intensive Process
Creating a high-quality KBr pellet requires care, skill, and time. The sample and KBr must be ground to an extremely fine powder to reduce light scattering, mixed homogeneously, and pressed carefully to create a clear, non-cracked pellet.
Potential for Sample Interaction
For certain types of samples, such as some inorganic salts, there is a risk of an ion-exchange reaction with the KBr matrix under pressure. This can alter the sample and produce an inaccurate spectrum.
Modern Alternatives to the KBr Method
Advances in instrumentation have provided powerful alternatives that bypass the challenges associated with KBr pellets.
Attenuated Total Reflectance (ATR)
ATR is now the most common technique for IR analysis of solids and liquids. It requires virtually no sample preparation.
The sample is simply pressed against a high-refractive-index crystal (often diamond or zinc selenide). The IR beam reflects within the crystal and a small portion of its energy penetrates the sample, generating a spectrum. This method is fast, easy, and non-destructive.
Nujol Mull
An older technique involves grinding the solid sample with a few drops of mineral oil (Nujol) to create a thick paste, or mull. This paste is then spread between two salt plates (which can be made of KBr or NaCl).
The primary disadvantage is that the mineral oil itself has C-H absorption bands that will always be present in the spectrum, potentially obscuring parts of the sample's fingerprint.
Making the Right Choice for Your Analysis
Choosing the correct sample preparation technique depends entirely on your sample, your equipment, and your analytical goal.
- If your primary focus is obtaining the highest quality, classic transmission spectrum for a solid sample: The KBr pellet method, when performed correctly in a dry environment, remains a gold standard.
- If your primary focus is speed, ease of use, and minimal sample preparation: Attenuated Total Reflectance (ATR) is almost always the superior modern choice for routine analysis.
- If your sample is sensitive to pressure or may react with KBr: Consider the Nujol mull technique or ATR as safer alternatives.
Understanding the principles behind your choice of matrix empowers you to generate clean, reliable, and interpretable spectroscopic data.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| IR Transparency | No covalent bonds = no interference in 4000–400 cm⁻¹ range |
| Hygroscopic Nature | Absorbs moisture, requiring careful drying to avoid water peaks |
| Pellet Formation | Creates clear matrix for solid samples under pressure |
| Refractive Index | Minimizes light scattering for cleaner baselines |
Need reliable IR spectroscopy solutions? KINTEK specializes in lab equipment and consumables for precise sample preparation and analysis. Whether you require KBr pellets, ATR accessories, or expert guidance to optimize your spectroscopic results, our products ensure accuracy and efficiency for your laboratory. Contact us today to discuss your specific needs and enhance your IR capabilities!
Visual Guide
Related Products
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press 25T 30T 50T
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Cold Isostatic Pressing Machine CIP for Small Workpiece Production 400Mpa
People Also Ask
- What types of ultra low temperature freezer models are available for space-limited labs? Optimize Your Lab's Layout and Storage
- What is the physical process of deposition? A Guide to PVD Thin-Film Coating
- Why is a thin film circuit important? Achieve Unmatched Precision for High-Frequency Electronics
- What process is used to extract essential oils? From Crude Plant to Pure Perfume
- What is sintered steel used for? Creating High-Performance, Complex Metal Components
- What are the advantages and disadvantages of heat treatment? Master Material Properties for Your Application
- What is the technology of thin film deposition? The Foundation of Modern Electronics and Materials
- What products are surface hardening? A Guide to Processes, Agents, and Applications



















