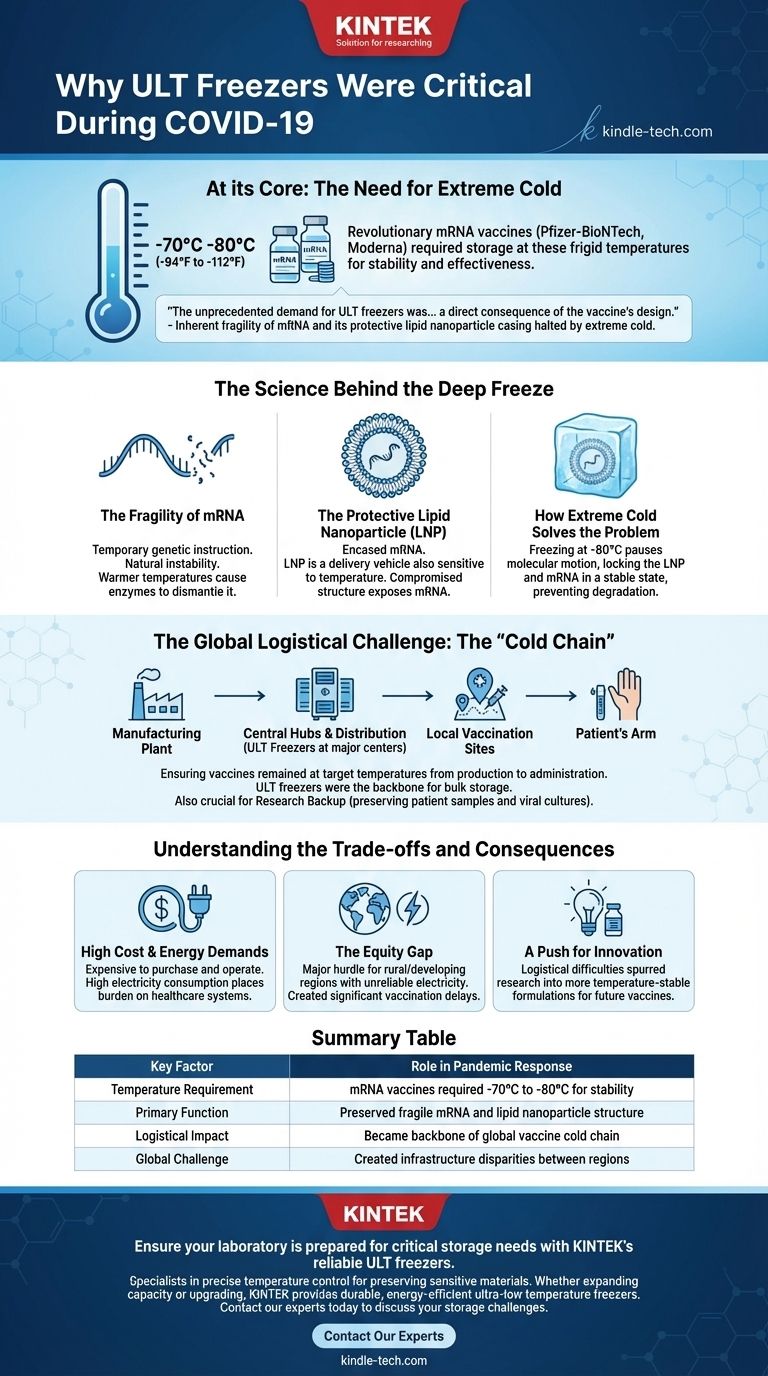
At its core, the global importance of Ultra-Low Temperature (ULT) freezers during the COVID-19 pandemic was driven by the specific needs of a new vaccine technology. The revolutionary mRNA vaccines, such as those from Pfizer-BioNTech and Moderna, required storage at frigid temperatures between -70°C and -80°C (-94°F to -112°F) to remain stable and effective. This requirement made ULT freezers an indispensable component of the worldwide vaccination effort.
The unprecedented demand for ULT freezers was not arbitrary; it was a direct consequence of the vaccine's design. The delicate messenger RNA (mRNA) and its protective lipid nanoparticle casing are inherently fragile, and extreme cold is the only way to halt the molecular degradation that would otherwise render the vaccine useless.

The Science Behind the Deep Freeze
To understand why these freezers were so critical, we must look at the fundamental biology of the vaccines themselves. Unlike traditional vaccines, which often use a weakened or inactivated virus, mRNA vaccines use a completely new and more delicate mechanism.
The Fragility of mRNA
Messenger RNA is a temporary genetic instruction. Inside our cells, its job is to deliver a message and then quickly break down. This natural instability is a significant hurdle when using mRNA as a drug.
At warmer temperatures, enzymes and chemical processes can rapidly dismantle the mRNA strand, destroying the instructions needed to provoke an immune response.
The Protective Lipid Nanoparticle (LNP)
To protect the fragile mRNA on its journey into human cells, scientists encased it in a microscopic bubble of fat called a lipid nanoparticle (LNP). This LNP acts as a delivery vehicle.
However, the LNP structure is also sensitive to temperature. If not kept sufficiently cold, its structure can be compromised, exposing the mRNA to premature degradation.
How Extreme Cold Solves the Problem
Freezing the vaccine to -80°C essentially pauses all molecular motion. It locks the LNP and the mRNA strand in a stable, frozen state.
This deep freeze prevents the enzymes from functioning and stops the chemical reactions that would otherwise break down the vaccine's active ingredients, ensuring it is fully potent when administered.
The Global Logistical Challenge: The "Cold Chain"
The scientific requirement for ultra-low temperatures created an immense logistical challenge: building and maintaining a global "cold chain." This meant ensuring the vaccines remained at their target temperature from the manufacturing plant to the patient's arm.
Central Hubs and Distribution
ULT freezers were deployed at centralized distribution hubs, major hospitals, and research centers. These locations served as the backbone of the cold chain, allowing for the bulk storage of millions of doses before they were sent to local vaccination sites.
Emergency and Research Backup
Beyond vaccine storage, these freezers also served a vital role in securing critical biological materials. During the pandemic, labs working on COVID-19 research relied on ULT freezers to preserve patient samples and viral cultures, preventing the loss of invaluable data in case of other equipment failures.
Understanding the Trade-offs and Consequences
The reliance on this specialized technology was not without its challenges and created significant disparities in the global response.
High Cost and Energy Demands
ULT freezers are expensive to purchase, maintain, and operate. They consume a significant amount of electricity, placing a heavy financial and infrastructural burden on healthcare systems.
The Equity Gap
The need for an unbroken ultra-cold chain created a major hurdle for rural and developing regions. Areas with unreliable electricity or without the resources to purchase and maintain fleets of ULT freezers faced significant delays and challenges in their vaccination campaigns.
A Push for Innovation
The logistical difficulties of the first-generation mRNA vaccines spurred intense research into creating more temperature-stable formulations. Future vaccine technologies aim to reduce or eliminate the need for such an extreme cold chain.
Key Takeaways for Different Contexts
Your understanding of the ULT freezer's role depends on your perspective.
- If your primary focus is public health logistics: The core challenge was building and maintaining an unprecedented global ultra-cold supply chain to ensure vaccine viability from factory to patient.
- If your primary focus is vaccine development: The primary scientific hurdle was stabilizing a naturally fragile mRNA molecule and its delivery system for mass distribution, a problem solved by extreme cold.
- If your primary focus is global health equity: The reliance on expensive ULT freezers highlighted the critical infrastructure gap between wealthy and developing nations, directly impacting the speed and success of vaccine rollouts.
Ultimately, the ULT freezer became an unassuming yet indispensable hero in the global fight against the pandemic, bridging the gap between cutting-edge science and public health.
Summary Table:
| Key Factor | Role in Pandemic Response |
|---|---|
| Temperature Requirement | mRNA vaccines required -70°C to -80°C for stability |
| Primary Function | Preserved fragile mRNA and lipid nanoparticle structure |
| Logistical Impact | Became backbone of global vaccine cold chain |
| Global Challenge | Created infrastructure disparities between regions |
Ensure your laboratory is prepared for critical storage needs with KINTEK's reliable ULT freezers.
As specialists in laboratory equipment, we understand the vital role that precise temperature control plays in preserving sensitive materials like vaccines, research samples, and biological reagents. Whether you're expanding your cold chain capacity or upgrading existing infrastructure, KINTEK provides the durable, energy-efficient ultra-low temperature freezers your lab requires.
Contact our experts today to discuss how we can support your specific laboratory storage challenges and help you maintain the integrity of your most valuable samples.
Visual Guide
Related Products
- 58L Precision Laboratory Ultra Low Temperature Upright Freezer for Critical Sample Storage
- 408L Advanced Vertical Laboratory Ultra Low Temperature Freezer for Critical Research Material Preservation
- 508L Advanced Vertical Ultra Low Temperature Freezer for Critical Laboratory Storage
- 608L Essential Laboratory Ultra Low Temperature Freezer For Critical Sample Preservation
- 708L Ultra Low Temperature Freezer High Performance Laboratory Freezer
People Also Ask
- What are the recommendations for storing mRNA vaccines in ultra-low temperature freezers? Ensure Absolute Stability at -80°C
- What is the role of an ultra-low temperature (ULT) freezer in the freeze-thaw synthesis of hydrogel nanocomposites?
- How do ultra-low temperature freezers achieve such low temperatures? The Science Behind -80°C Cooling
- How do ultra-low temperature freezers work? Unlocking the Secrets of -86°C Sample Preservation
- What features do ultra-low temperature freezers typically include? Ensuring Absolute Sample Security



















