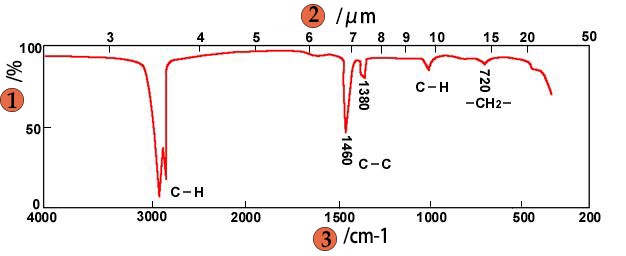
Introduction to Sample Preparation for Infrared Spectroscopy
Importance of Correct Sample Preparation
Correct sample preparation is paramount for obtaining high-quality infrared spectra. The choice of preparation method is contingent upon the specific characteristics of the sample and the objectives of the experiment. Improper preparation can lead to inaccurate results, masking the true nature of the sample.
To ensure the reliability of your analysis, it is essential to reduce heterogeneity within the sample. This means that the sample should be representative of the population being studied, thereby eliminating any variability that could skew the results. Consistency in preparation is key to achieving reproducible results, as even minor deviations can introduce significant variability.
Moreover, proper sample preparation helps to eliminate interferences from impurities and contaminants. These unwanted elements can distort the spectral data, leading to erroneous conclusions. By meticulously preparing the sample, you can enhance the sensitivity of the analysis, enabling the detection of trace levels of analytes that might otherwise go unnoticed.
In summary, the correct sample preparation method not only ensures the accuracy and reliability of your infrared spectra but also enhances the overall quality of your analytical results.
Specific Sample Preparation Methods
Potassium Bromide Pressing Method
The potassium bromide pressing method is a widely adopted technique for preparing solid powder samples for infrared spectroscopy. This method involves several key steps to ensure the sample is transformed into a form suitable for accurate spectral analysis.

Firstly, the solid powder sample is meticulously mixed with potassium bromide (KBr) powder. This mixture is crucial as potassium bromide is an inert, transparent material in the infrared region, which allows for minimal interference during spectral analysis. The ratio of the sample to KBr is typically maintained at a low concentration, often around 1-2%, to ensure that the sample's spectral features are not overshadowed by the KBr.
Next, the mixed powder is subjected to a pressing process. This is usually done using a hydraulic press, which applies high pressure to the mixture. The pressure exerted is significant, often ranging from 7 to 10 tons, to compact the powder into a dense, transparent ingot tablet. The formation of this tablet is essential as it provides a uniform, flat surface that is ideal for infrared spectroscopy.
The resulting ingot tablet is then ready for infrared spectral analysis. The transparency of the tablet ensures that the infrared light can pass through it without significant scattering or absorption by the KBr, allowing for precise detection of the sample's spectral characteristics. This method is particularly advantageous for samples that are difficult to dissolve or those that require minimal sample manipulation, thereby preserving the integrity of the sample's structure and properties.
In summary, the potassium bromide pressing method is a robust and reliable technique for preparing solid powder samples for infrared spectroscopy. By carefully mixing the sample with KBr and pressing it into a transparent tablet, this method ensures high-quality spectral data, making it a preferred choice in many analytical laboratories.
Halide Crystal Coating Method
The halide crystal coating method is a specialized technique tailored for the direct measurement of liquid samples, particularly those that are uncured and possess a viscous consistency, such as resins and inks. This method involves the meticulous application of the liquid sample onto a halide wafer, which serves as a transparent substrate for infrared spectroscopy.
One of the primary advantages of this method is its simplicity and efficiency, making it an ideal choice for laboratories and research facilities where rapid analysis is crucial. Unlike other methods that require complex preparation steps or the use of additional reagents, the halide crystal coating method allows for direct application and immediate measurement, thereby saving time and reducing potential sources of error.
The choice of halide wafer material, typically sodium chloride or potassium bromide, is critical as these materials are highly transparent to infrared light, ensuring that the resulting spectra are clear and accurate. The wafer acts as a medium that facilitates the transmission of infrared radiation, enabling the detection of specific molecular vibrations that are characteristic of the sample's chemical composition.
Moreover, this method is particularly effective for samples that are difficult to handle using traditional solid-state preparation techniques. The ability to coat viscous liquids directly onto the halide wafer eliminates the need for additional processing steps, such as drying or grinding, which can alter the sample's properties and compromise the integrity of the data.
In summary, the halide crystal coating method offers a straightforward and reliable approach for the analysis of uncured viscous resins and inks, providing researchers with a valuable tool for obtaining high-quality infrared spectra with minimal sample preparation.
Cracking Method
The cracking method is specifically tailored for thermosetting resins and crosslinked polymers, which are notoriously difficult to analyze due to their complex molecular structures and high degree of crosslinking. This technique involves heating the sample to a temperature sufficient to induce thermal cracking, thereby breaking down the polymer chains into smaller, more manageable fragments.
Upon reaching the critical temperature, the polymer undergoes a controlled decomposition process, yielding a liquid phase that is rich in volatile components and lower molecular weight species. This liquid is then carefully collected and applied as a thin, uniform layer onto a sodium chloride wafer. The sodium chloride wafer, with its smooth, flat surface, provides an ideal substrate for infrared spectroscopy, ensuring that the resulting spectrum is clear and interpretable.
This method is particularly advantageous for samples that are highly crosslinked or otherwise resistant to conventional sample preparation techniques. By transforming the sample into a more accessible form, the cracking method allows for detailed analysis of the polymer's chemical composition and structural features, providing valuable insights into its properties and behavior.
Potassium Bromide Triangular Enrichment Method
The Potassium Bromide Triangular Enrichment Method is specifically designed for trace samples that contain minimal amounts of inorganic impurities. This technique leverages the unique properties of potassium bromide, which is known for its high purity and transparency in the infrared spectrum.
In this method, the sample is meticulously enriched on a specially designed potassium bromide triangular block. This block serves as both a concentrator and a substrate, allowing for the precise accumulation of trace elements. The triangular shape of the block not only facilitates efficient sample distribution but also ensures uniform enrichment, which is crucial for obtaining accurate and reproducible infrared spectra.
Key advantages of this method include its sensitivity to low concentrations of impurities and its ability to handle small sample sizes effectively. The process is particularly useful in analytical chemistry, where the detection of trace elements is paramount. By concentrating the sample on the potassium bromide block, researchers can enhance the signal-to-noise ratio, thereby improving the accuracy of their spectroscopic analyses.
Moreover, the Potassium Bromide Triangular Enrichment Method is versatile and can be adapted to various experimental conditions. Whether the sample is a solid, liquid, or gas, this technique offers a reliable means of preparation, ensuring that the resulting spectra are of high quality and informative.
Reflection Method (ATR)
The Attenuated Total Reflection (ATR) method is particularly well-suited for the analysis of thin coatings and materials that require non-destructive testing. This technique leverages the principles of infrared spectroscopy to provide detailed insights into the chemical composition and structure of samples without altering their physical state.
In ATR spectroscopy, a beam of infrared light is directed into an internal reflection element, typically made of a high refractive index material such as zinc selenide or germanium. When the light enters the element at a specific angle, it undergoes total internal reflection at the interface with the sample. During this process, a portion of the light penetrates into the sample, known as the evanescent wave, which interacts with the sample's molecules. This interaction causes the light to be attenuated, providing a spectrum that can be analyzed to determine the sample's composition.
One of the key advantages of the ATR method is its ability to analyze samples that are difficult to prepare using traditional techniques. For instance, it can be used to study the properties of coatings on various substrates, such as paints on metal surfaces or polymer films on glass. The non-destructive nature of ATR makes it an ideal choice for quality control and forensic analysis, where preserving the integrity of the sample is crucial.
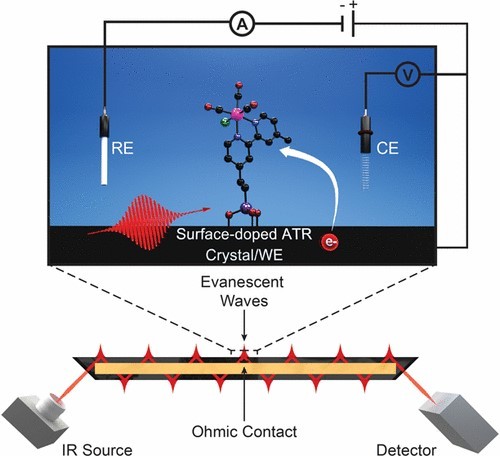
Moreover, the ATR method is highly versatile and can be applied to a wide range of sample types, including liquids, solids, and gases. This versatility, combined with its ease of use and minimal sample preparation requirements, makes ATR a valuable tool in both research and industrial settings.
Hot Pressing Method
The hot pressing method is a sophisticated technique that integrates press molding and heat sintering into a single, simultaneous process. This method is particularly advantageous for studying changes in the crystallinity of polymers, as it allows for precise control over the sample's structural integrity and density.
During hot pressing, the polymer sample is subjected to both heat and pressure, typically within stainless steel molds. The thermoplastic state of the polymer under these conditions significantly reduces its deformation resistance, facilitating easy plastic flow and densification. This ease of deformation means that the required molding pressure is relatively low, making the process both efficient and cost-effective.
One of the key benefits of hot pressing is its ability to enhance the contact, diffusion, and flow between polymer particles. This interaction not only lowers the sintering temperature but also shortens the sintering time, effectively suppressing the growth of crystal grains. As a result, the method can produce sintered bodies that are nearly at theoretical density, with porosity approaching zero and a fine grain structure.
| Advantage | Description |
|---|---|
| Low Deformation Resistance | The thermoplastic state of the polymer reduces the need for high molding pressures. |
| Enhanced Particle Interaction | Simultaneous heating and pressurization improve contact, diffusion, and flow between particles. |
| Suppressed Grain Growth | The process lowers sintering temperatures and times, preventing excessive grain growth. |
| High Density and Fine Grain Structure | Produces sintered bodies close to theoretical density with minimal porosity and fine grains. |
The hot pressing process involves compacting a powder part under pressure while applying heat, ensuring good mechanical properties and dimensional accuracy. A controlled atmosphere is essential to maintain the integrity of the process, and mold materials must withstand extreme temperature and pressure conditions. The choice of mold material, such as super alloys or graphite, depends on the specific powder material being processed, particularly for refractory metals.
Related Products
- No Demolding Lab Infrared Press Mold for Laboratory Applications
- Lab Infrared Press Mold
- XRF & KBR plastic ring lab Powder Pellet Pressing Mold for FTIR
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use