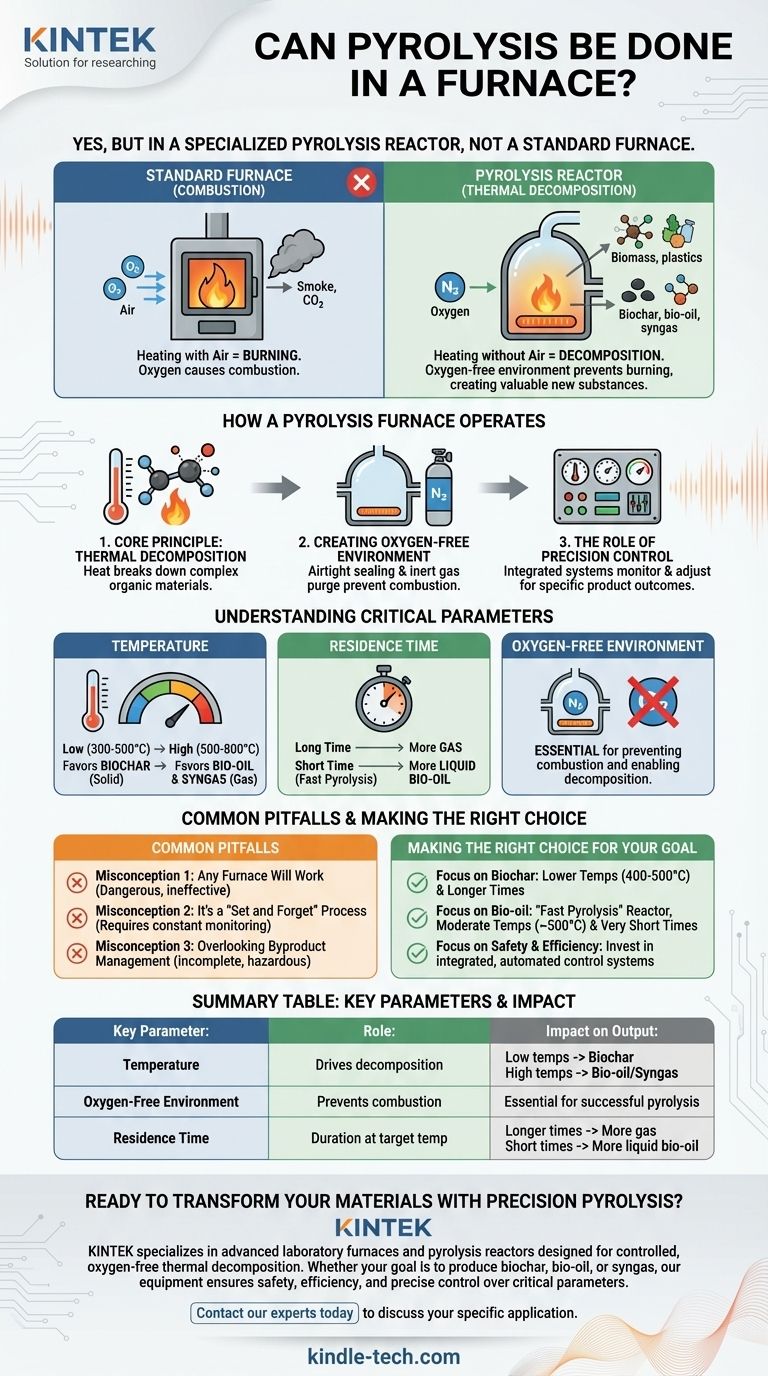
Yes, pyrolysis is fundamentally a furnace-based process. However, it's not performed in just any furnace. It requires a specialized, sealed chamber designed to heat organic material to very high temperatures in a controlled, oxygen-free environment to trigger thermal decomposition rather than combustion.
A standard furnace is designed for heating in the presence of air, which causes burning. A pyrolysis furnace, more accurately called a pyrolysis reactor, is a highly controlled system engineered to prevent burning by eliminating oxygen, thereby breaking material down into valuable new substances.

How a Pyrolysis Furnace Operates
A pyrolysis furnace is the heart of a system designed for a specific chemical transformation. Its operation hinges on precise control over the reaction environment.
The Core Principle: Thermal Decomposition
Pyrolysis is the process of breaking down complex organic materials, like biomass or plastics, using heat. Unlike burning, which is a chemical reaction with oxygen, pyrolysis is a thermal decomposition.
The furnace's heating elements raise the temperature of the material inside the chamber to a point where the chemical bonds within the molecules become unstable and break apart.
Creating the Oxygen-Free Environment
This is the single most critical factor that distinguishes a pyrolysis furnace from a standard one. Oxygen must be absent to prevent the material from simply catching fire (combustion).
To achieve this, the furnace chamber is sealed airtight. Before heating begins, any oxygen is often purged from the system and replaced with an inert gas, like nitrogen. This ensures that only heat acts upon the material.
The Role of Precision Control
The furnace is not just a hot box; it is an integrated system. Sophisticated controls constantly monitor and adjust key parameters to dictate the outcome of the process. The final products—gas, liquid, or solid—depend entirely on these settings.
Understanding the Critical Parameters
The efficiency of pyrolysis and the specific products you create are determined by three main variables. Controlling these is essential for achieving a desired outcome.
Temperature
Temperature is the primary driver of the reaction. Different temperature ranges favor different products.
- Low Temperatures (300-500°C): Slower heating at lower temperatures tends to maximize the yield of the solid residue, known as biochar.
- High Temperatures (500-800°C): Faster heating at higher temperatures favors the production of liquids (bio-oil) and combustible gases (syngas).
Residence Time
This refers to how long the material is kept at the target temperature inside the furnace. A longer residence time allows the thermal decomposition to proceed further, breaking down larger molecules into smaller ones.
Longer times can increase gas production, while very short residence times are often used in "fast pyrolysis" to maximize liquid bio-oil yield.
Pressure and Monitoring
While temperature and time are primary, pressure within the sealed furnace must also be managed for safety and process stability. Integrated control loops monitor these conditions to prevent dangerous buildups and ensure the reaction proceeds as planned. Modern systems use predictive models to anticipate and correct issues before they occur.
Common Pitfalls to Avoid
Understanding the difference between a simple heater and a pyrolysis reactor is crucial for safety and success.
Misconception 1: Any Furnace Will Work
A standard industrial furnace or kiln is designed for processes that occur in the presence of air. Using one for pyrolysis without extensive modification is ineffective and extremely dangerous, as the hot gases produced are flammable and can ignite if oxygen is present.
Misconception 2: It's a "Set and Forget" Process
Pyrolysis is a dynamic process. The composition of the feedstock and the progress of the reaction require constant monitoring and adjustment. Safe operation relies on reliable control systems and, in industrial settings, smart technologies to maintain stability.
Misconception 3: Overlooking Byproduct Management
The process creates a mixture of solids, liquids, and gases. A complete pyrolysis system includes equipment to safely collect, cool, and separate these outputs. Simply heating material in a sealed drum without this back-end infrastructure is an incomplete and hazardous approach.
Making the Right Choice for Your Goal
The design and operation of a pyrolysis furnace are dictated by the intended product.
- If your primary focus is producing biochar for agriculture: You will need a system optimized for lower temperatures (around 400-500°C) and longer residence times to maximize the solid output.
- If your primary focus is creating bio-oil as a liquid fuel: You will need a "fast pyrolysis" reactor designed for rapid heating to moderate temperatures (around 500°C) with a very short residence time.
- If your primary focus is industrial safety and efficiency: You must invest in a furnace with an integrated, automated control loop to precisely manage temperature, pressure, and feedstock flow.
By controlling the furnace environment with precision, you transform a simple heating process into a sophisticated method for chemical manufacturing.
Summary Table:
| Key Parameter | Role in Pyrolysis | Impact on Output |
|---|---|---|
| Temperature | Drives the thermal decomposition reaction. | Low temps (300-500°C) favor biochar; high temps (500-800°C) favor bio-oil/syngas. |
| Oxygen-Free Environment | Prevents combustion, enabling decomposition. | Essential for successful pyrolysis; absence of oxygen is critical. |
| Residence Time | Duration material is held at target temperature. | Longer times increase gas yield; short times maximize liquid bio-oil. |
Ready to transform your materials with precision pyrolysis?
KINTEK specializes in advanced laboratory furnaces and pyrolysis reactors designed for controlled, oxygen-free thermal decomposition. Whether your goal is to produce biochar, bio-oil, or syngas, our equipment ensures safety, efficiency, and precise control over critical parameters.
Contact our experts today to discuss your specific application and discover the right pyrolysis solution for your lab.
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Does heat treatment affect chemical composition? Master the Science of Material Properties
- What is the temperature range for quenching? Achieve Perfect Hardness for Your Steel Alloy
- What is conduction in vacuum? Understanding Heat Transfer in the Absence of Matter
- What are the advantages of SPS furnaces for UHTCMCs? Achieve Superior Density and Microstructure
- Which furnace has the highest temperature? Exploring the Limits of Extreme Heat
- What happens to heat generated in a vacuum? Mastering Thermal Control for Superior Materials
- Is physical vapor deposition safe? Understanding the Engineered Safety of PVD Technology
- What are the advantages of electric arc furnace over blast furnace? Boost Efficiency & Sustainability



















