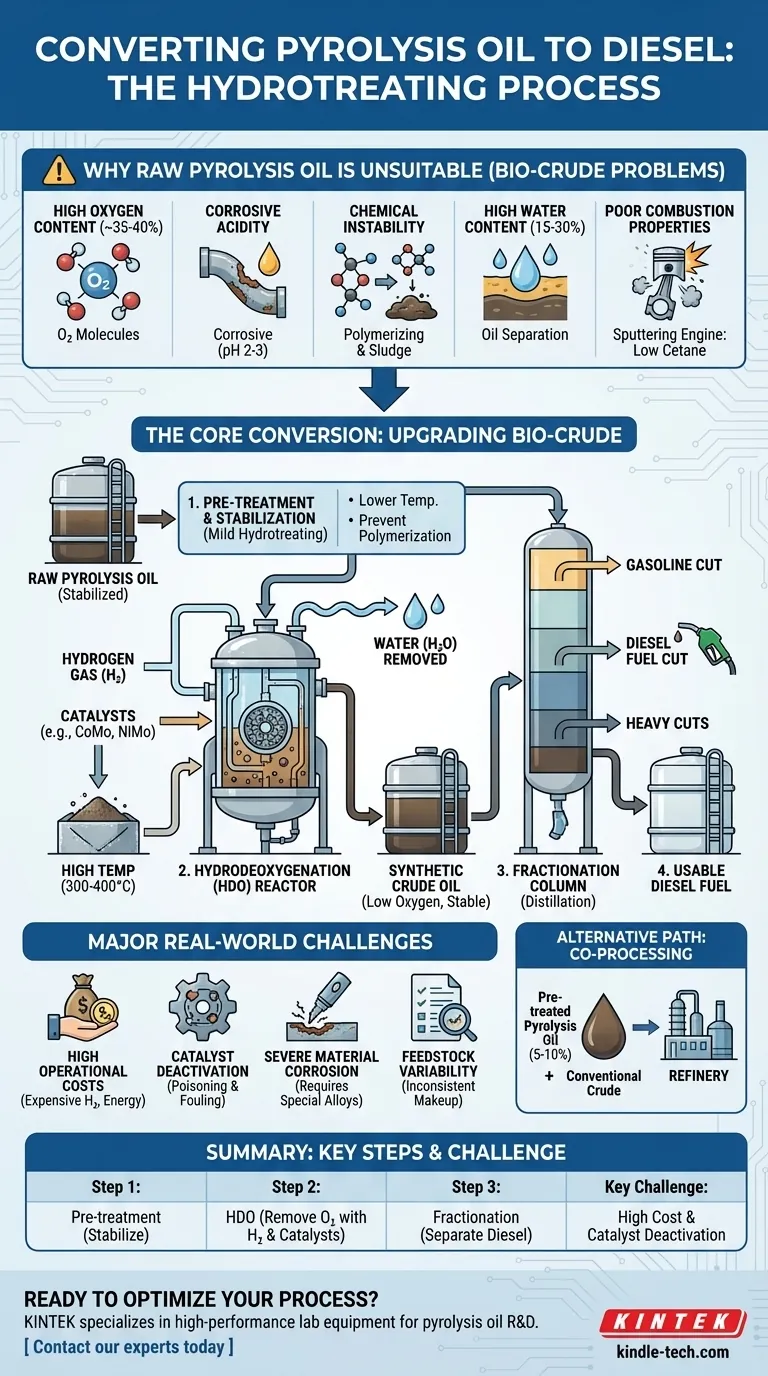
In short, you convert pyrolysis oil to diesel primarily through a high-pressure, high-temperature catalytic process called hydrotreating, or more specifically, hydrodeoxygenation (HDO). This chemical process uses hydrogen to remove the high oxygen content and other impurities that make raw pyrolysis oil acidic, unstable, and unsuitable for use as a direct replacement for diesel fuel. The process is complex and requires significant pre-treatment and post-treatment stages.
The core challenge is not simply converting the oil, but overcoming its fundamentally corrosive and unstable nature. Raw pyrolysis oil is chemically very different from crude oil, and upgrading it to a stable, usable diesel fuel is an energy-intensive and expensive process fraught with technical hurdles.

Why Pyrolysis Oil Isn't Diesel (Yet)
To understand the conversion process, you must first understand why it's necessary. Raw pyrolysis oil, often called bio-crude, cannot be used in a standard diesel engine because of its inherent chemical properties.
High Oxygen Content
Pyrolysis oil contains a very high concentration of oxygen (often 35-40%), locked in compounds like acids, aldehydes, and phenols. This is the root cause of many other problems.
Corrosive Acidity
The oxygenated compounds, particularly acetic and formic acids, make the oil highly acidic (pH 2-3). This level of acidity will rapidly corrode standard storage tanks, pipelines, and engine components.
Chemical Instability
Pyrolysis oil is thermally unstable. Over time, or when heated, its molecules react with each other in a process called polymerization. This forms thick gums and sludge that will clog filters, fuel lines, and injectors.
High Water Content
The oil is often produced with a significant amount of water (15-30%), which is mixed in with the oil. This lowers its energy density and can cause issues in combustion.
Poor Combustion Properties
Compared to diesel, pyrolysis oil has a very low cetane number, meaning it does not ignite readily under compression. This results in poor engine performance and high emissions.
The Core Conversion Process: Upgrading Bio-Crude
Upgrading pyrolysis oil is a multi-stage process designed to solve the problems listed above. The central goal is to remove oxygen and stabilize the fuel.
Step 1: Pre-treatment and Stabilization
Before the main conversion, the raw oil often undergoes a stabilization step. This is a mild form of hydrotreating at lower temperatures to convert the most reactive aldehydes and prevent polymerization during storage and heating.
Step 2: Hydrodeoxygenation (HDO)
This is the heart of the conversion. The stabilized oil is fed into a high-pressure reactor with a stream of hydrogen gas at high temperatures (300-400°C) and pressures (100-200 bar).
In the presence of a specialized catalyst, the hydrogen reacts with the oil. This reaction breaks the carbon-oxygen bonds, removing oxygen in the form of water (H₂O). It also removes other contaminants like sulfur and nitrogen.
Step 3: The Role of Catalysts
The process is impossible without catalysts. These are typically sulfide-based catalysts like Cobalt-Molybdenum (CoMo) or Nickel-Molybdenum (NiMo) on an alumina support, similar to those used in conventional oil refineries. Catalyst performance and lifespan are critical technical and economic factors.
Step 4: Fractionation
The output from the HDO reactor is a synthetic crude oil, now low in oxygen and much more stable. This synthetic crude is then fed into a distillation column (a process called fractionation) where it is separated by boiling point into different fuel cuts, including a diesel-range fraction.
Understanding the Trade-offs and Challenges
While technically feasible, converting pyrolysis oil to diesel faces significant real-world challenges that have limited its commercial deployment.
High Operational Costs
The HDO process requires vast amounts of hydrogen, which is expensive to produce. The high pressures and temperatures also demand significant energy input, adding to the operational expense.
Catalyst Deactivation
The residual contaminants and acidic nature of even pre-treated pyrolysis oil can quickly "poison" and deactivate the expensive catalysts. This shortens their lifespan, requiring frequent and costly replacement and causing operational downtime.
Severe Material Corrosion
Because of the oil's acidity, the reactors, piping, and other equipment must be constructed from expensive, corrosion-resistant stainless steel or other alloys, dramatically increasing the plant's capital cost.
Feedstock Variability
The exact chemical makeup of pyrolysis oil changes depending on the feedstock used (e.g., wood, agricultural waste, plastic). This variability makes it difficult to maintain a stable and optimized upgrading process.
An Alternative Path: Co-processing
A more economically viable, near-term approach is co-processing. In this model, a small amount of pre-treated pyrolysis oil (typically 5-10%) is blended directly into a feed stream at a conventional oil refinery. This leverages existing infrastructure, but the percentage is limited by the negative impact of the oil's contaminants on the refinery's primary catalysts.
Making the Right Choice for Your Goal
Your approach to converting pyrolysis oil depends entirely on your objective.
- If your primary focus is research and development: Concentrate on creating novel, low-cost catalysts that are more resistant to deactivation and developing more efficient pre-treatment methods to stabilize the oil.
- If your primary focus is commercial viability: Investigate co-processing with a partner refinery as the most pragmatic path to market, as building a dedicated, standalone upgrading plant carries immense financial risk.
- If your primary focus is environmental impact: Acknowledge that while it promotes a circular economy, the upgrading process itself is energy and resource-intensive, and its overall carbon footprint must be carefully analyzed.
Ultimately, transforming pyrolysis oil into diesel is a battle against chemistry, and success requires a deep understanding of the technical challenges and economic realities involved.
Summary Table:
| Step | Process | Key Objective |
|---|---|---|
| 1 | Pre-treatment & Stabilization | Convert reactive aldehydes to prevent polymerization |
| 2 | Hydrodeoxygenation (HDO) | Remove oxygen using hydrogen & catalysts (300-400°C, 100-200 bar) |
| 3 | Fractionation | Separate upgraded oil into diesel and other fuel cuts |
| Key Challenge | High Cost & Catalyst Deactivation | Requires expensive hydrogen and corrosion-resistant equipment |
Ready to optimize your fuel conversion process? KINTEK specializes in high-performance lab equipment and consumables essential for pyrolysis oil research and development. Whether you're testing novel catalysts or scaling up your pre-treatment methods, our reliable tools help you tackle technical challenges efficiently. Contact our experts today to find the right solutions for your laboratory's needs.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds