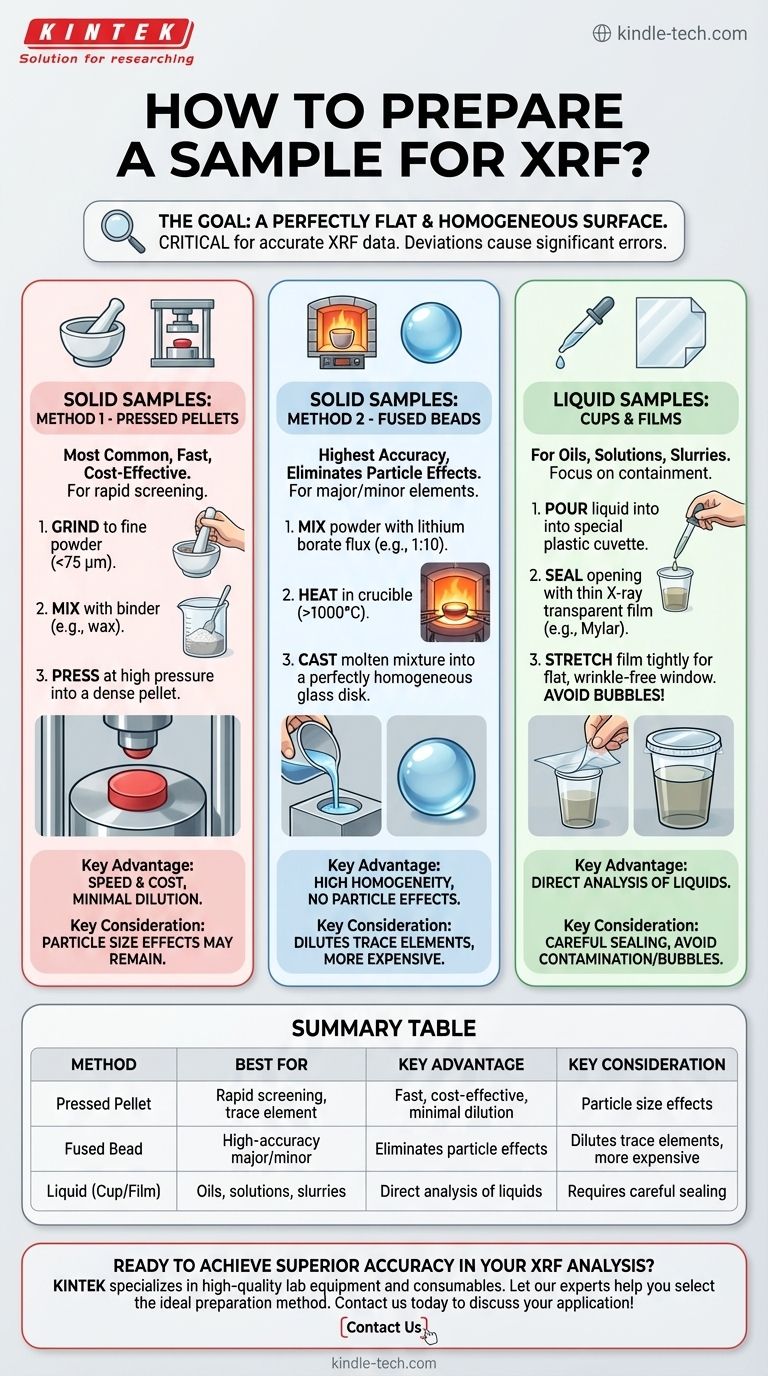
To prepare a sample for XRF, you must convert it into a form with a perfectly flat and homogeneous surface. For solid materials, this typically involves grinding the sample into a fine powder (<75 µm) and either pressing it into a pellet or fusing it with a flux to create a glass-like bead. Liquid samples are analyzed in a special cup sealed with a thin, transparent film.
The single most important goal of XRF sample preparation is to create a sample that is homogeneous and has a perfectly flat surface. Any deviation in composition or surface topography will introduce significant errors, as XRF systems are calibrated for a fixed distance and assume the measured area represents the entire sample.

The Foundation of Accurate XRF: Why Preparation Matters
The quality of your XRF data is determined before the analysis even begins. Proper preparation is not an optional step; it is the foundation of a reliable measurement.
Achieving Homogeneity
A fundamental assumption in XRF is that the small area being analyzed is perfectly representative of the entire bulk sample.
Grinding, pressing, and fusing are all methods designed to eliminate variations in the material, ensuring the X-ray beam interacts with a uniform matrix.
The Critical Role of a Flat Surface
XRF instruments measure the intensity of X-rays emitted from the sample, which is highly dependent on the distance between the sample and the detector.
An irregular or non-flat surface creates variations in this distance, causing some elements to appear more or less concentrated than they truly are. This is one of the largest sources of analytical error.
Ensuring Mechanical Stability
The prepared sample, whether a pellet or a cuvette, must be robust enough to be handled and placed in the spectrometer without breaking, crumbling, or leaking.
For pressed powders, binders are often used to impart the necessary mechanical strength to the pellet.
Preparing Solid Samples: The Two Primary Methods
For rocks, minerals, cements, metals, and other solids, your choice is almost always between a pressed pellet or a fused bead.
Method 1: Pressed Pellets
This is the most common method due to its speed, low cost, and excellent results for many applications.
The process involves crushing and grinding the raw material into a fine powder, ideally with a particle size below 75 microns. This powder is then often mixed with a wax-based binder and pressed under high pressure in a die to form a dense, solid pellet.
Method 2: Fused Beads
This method offers the highest level of accuracy by completely eliminating particle size and mineralogical effects.
The powdered sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C. The molten mixture dissolves the sample completely and is then cast into a mold to cool, forming a perfectly homogeneous glass disk.
Preparing Liquid Samples
Analyzing liquids like oils, solutions, or slurries requires a different approach focused on containment.
Using Sample Cups and Films
Liquids are poured into a specialized plastic cuvette or sample cup. The opening of this cup is sealed with a thin X-ray transparent film, such as Mylar or Polypropylene.
This film becomes the analytical surface. It must be stretched tightly over the cup to create a flat, wrinkle-free window and prevent sagging.
Key Precautions for Liquids
Before use, always check the film for impurities, as some may contain elements (like Si or Cl) that could interfere with the analysis.
Care must also be taken to avoid trapping air bubbles under the film, as this will create an uneven surface and compromise the results.
Understanding the Trade-offs: Pellets vs. Fused Beads
Choosing between a pressed pellet and a fused bead involves balancing your analytical needs for speed, cost, and accuracy.
Speed and Cost: The Advantage of Pellets
Pressed pellets are significantly faster and cheaper to prepare. The equipment is less expensive, and the process from raw sample to finished pellet can take just a few minutes.
Accuracy and Homogeneity: The Strength of Fused Beads
Fused beads are the gold standard for accuracy, especially for major and minor elements. The fusion process completely destroys the original crystal structure of the sample, creating a near-perfectly homogeneous medium free from particle-size effects.
The Dilution Effect on Trace Elements
The primary drawback of fusion is dilution. By mixing the sample with a flux (often at a 10:1 ratio), the concentration of every element is reduced. This can make it difficult or impossible to accurately measure elements present at very low (trace) levels.
Making the Right Choice for Your Goal
Your analytical objective dictates the correct preparation method.
- If your primary focus is rapid, cost-effective screening: Pressed pellets are the ideal choice, offering a great balance of speed and quality.
- If your primary focus is the highest possible accuracy for major elements: Fused beads are superior as they eliminate particle size effects, though they require more effort and expense.
- If your primary focus is analyzing trace elements: A carefully prepared pressed pellet is often preferred to avoid the dilution effect inherent in the fusion method.
- If your primary focus is analyzing liquids or oils: The film and cuvette method is your standard, but meticulous care is needed to avoid contamination and surface inconsistencies.
Proper sample preparation is not just a preliminary step; it is the foundation upon which all reliable XRF analysis is built.
Summary Table:
| Method | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Pressed Pellet | Rapid screening, trace element analysis | Fast, cost-effective, minimal dilution | Particle size effects may remain |
| Fused Bead | High-accuracy major/minor element analysis | Eliminates particle size effects, highly homogeneous | Dilutes trace elements, more expensive |
| Liquid (Cup/Film) | Oils, solutions, slurries | Direct analysis of liquids | Requires careful sealing to avoid bubbles/contamination |
Ready to achieve superior accuracy in your XRF analysis?
The right sample preparation is critical for reliable results. KINTEK specializes in high-quality lab equipment and consumables for XRF, including pellet presses, fusion fluxers, and sample cups. Our products are designed to help you create perfectly homogeneous and flat samples, ensuring your data is accurate and trustworthy.
Let our experts help you select the ideal preparation method and equipment for your specific laboratory needs. Contact us today to discuss your application!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
People Also Ask
- Why is a hydraulic pellet press used for FTIR? Transform Nanofillers into Clear Data
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- What is the function of a laboratory hydraulic press for Li10GeP2S12 pellets? Optimize Solid-State Battery Performance
- Why is a laboratory hydraulic press utilized for electrolyte pelletizing? Unlock High Ionic Conductivity
- What is KBr disc method? A Complete Guide to IR Spectroscopy Sample Prep



















