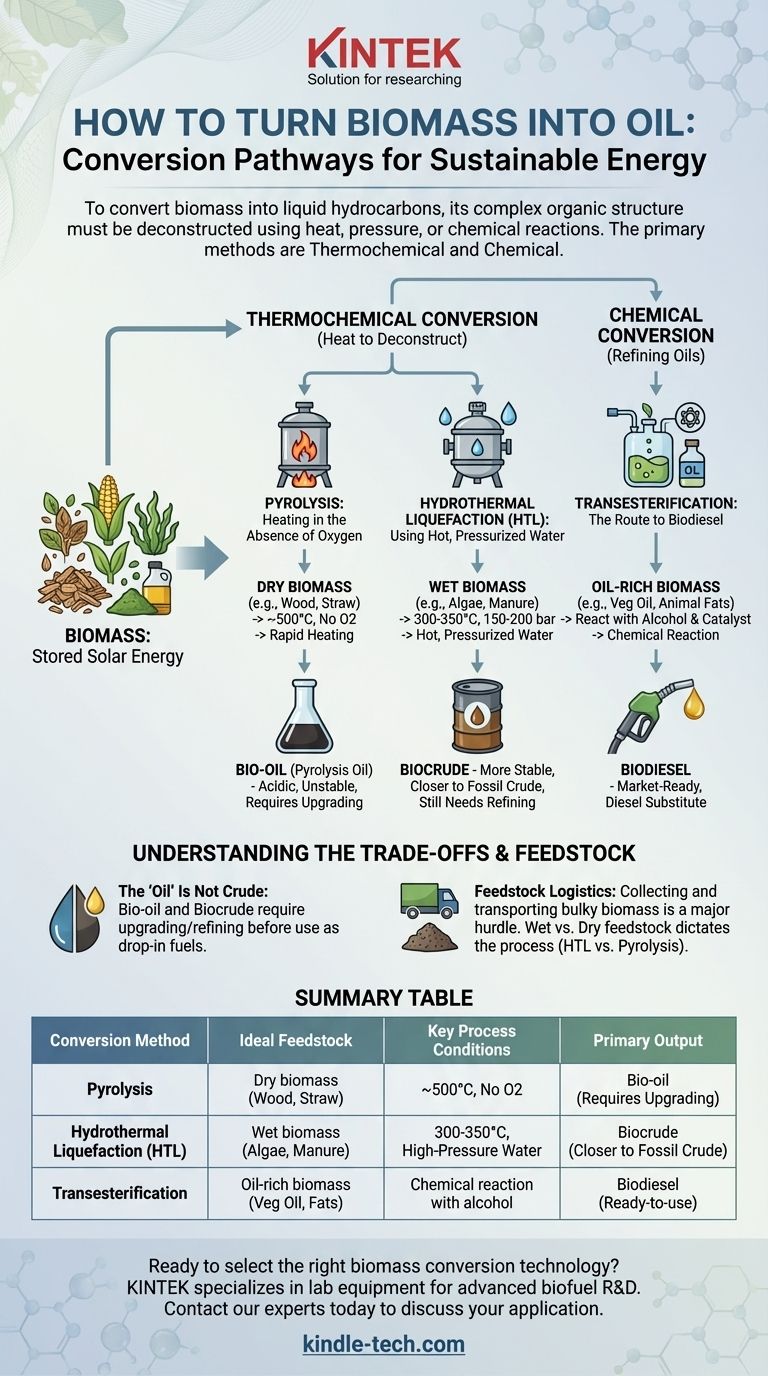
To turn biomass into oil, you must break down its complex organic structure into simpler, liquid hydrocarbon molecules. The primary methods for this are thermochemical processes like pyrolysis and hydrothermal liquefaction (HTL), which use intense heat and pressure to decompose the raw biomass. For specific fat-rich biomass, a chemical process called transesterification is used to create biodiesel.
The specific method used to create bio-oil is determined entirely by the type of biomass feedstock. Thermochemical conversion is used for raw plant matter, while specific chemical pathways are required for existing fats and oils.

The Core Conversion Pathways
Biomass is simply stored solar energy locked within organic matter. To release it as a liquid fuel, we must reverse the process of photosynthesis and deconstruct the plant material. This is accomplished through two main families of processes: thermochemical and chemical conversion.
Thermochemical: Using Heat to Deconstruct Biomass
This approach uses high temperatures to break down the complex polymers in biomass, like cellulose and lignin.
Pyrolysis: Heating in the Absence of Oxygen
Pyrolysis involves rapidly heating dry biomass (e.g., wood chips, corn stover, switchgrass) to around 500°C (932°F) in a reactor with no oxygen. Preventing oxygen from entering is critical to ensure the biomass does not simply burn.
This process thermally cracks the long organic molecules into smaller, volatile compounds. As these compounds cool, they condense into a dark, viscous liquid known as bio-oil or pyrolysis oil.
Hydrothermal Liquefaction (HTL): Using Hot, Pressurized Water
Hydrothermal liquefaction is ideally suited for wet biomass like algae, manure, or sewage sludge. It mimics the natural geological processes that form crude oil, but accomplishes it in minutes rather than millions of years.
In HTL, the wet feedstock is placed in a reactor with water at high temperatures (300-350°C) and high pressure (150-200 bar). At this state, water acts as a powerful solvent and catalyst, breaking down the biomass into a liquid biocrude that is more stable and energy-dense than pyrolysis oil.
Chemical: Refining Oils into Biodiesel
This pathway does not start with raw, fibrous biomass but with a specific type that is already rich in oils or fats (triglycerides).
Transesterification: The Route to Biodiesel
Transesterification is a well-established chemical reaction, not a decomposition process. It is used to convert vegetable oils, animal fats, or used cooking grease into biodiesel.
In this process, the oil is reacted with an alcohol (typically methanol) in the presence of a catalyst. The reaction breaks the large triglyceride molecules into smaller fatty acid methyl esters (biodiesel) and a co-product, glycerin.
Understanding the Trade-offs
Creating oil from biomass is a powerful concept, but it is not a simple replacement for drilling for fossil fuels. The quality of the output and the complexity of the process present significant challenges.
The "Oil" Is Not Crude Oil
The liquid produced from pyrolysis and HTL is not a drop-in substitute for the crude oil that goes into a conventional refinery.
Pyrolysis bio-oil is highly acidic, corrosive, and unstable, degrading over time. It also contains significant amounts of water and oxygen, which lower its energy content and require substantial upgrading (a form of pre-refining) before it can be used.
HTL biocrude is of higher quality, with less oxygen and more stability, making it closer to fossil crude. However, it still requires refining to remove impurities and be converted into usable fuels like gasoline or diesel.
Feedstock Is Everything
The biggest challenge in bio-oil production is logistics. Biomass is bulky, has low energy density, and is often geographically dispersed.
Collecting, transporting, and preparing massive quantities of wood, agricultural waste, or algae to feed a large-scale conversion plant is a major economic and energetic hurdle. The choice of HTL for wet feedstock is critical because the energy required to dry it for pyrolysis would make the process inefficient.
Making the Right Choice for Your Goal
The optimal conversion pathway is dictated by your feedstock and your desired end-product.
- If your primary focus is utilizing dry waste like wood chips or agricultural straw: Pyrolysis is the most direct thermochemical route to produce a raw bio-oil that can be upgraded into fuel.
- If your primary focus is converting wet feedstocks like algae, manure, or sewage sludge: Hydrothermal Liquefaction (HTL) is the most efficient method, as it avoids the massive energy penalty of drying the material.
- If your primary focus is creating a high-quality diesel substitute from vegetable oils or waste fats: Transesterification is the established and direct chemical pathway to produce market-ready biodiesel.
Understanding these distinct pathways is the first step toward leveraging biomass as a viable component of a future energy portfolio.
Summary Table:
| Conversion Method | Ideal Feedstock | Key Process Conditions | Primary Output |
|---|---|---|---|
| Pyrolysis | Dry biomass (wood chips, straw) | ~500°C, no oxygen | Bio-oil (requires upgrading) |
| Hydrothermal Liquefaction (HTL) | Wet biomass (algae, manure) | 300-350°C, high-pressure water | Biocrude (closer to fossil crude) |
| Transesterification | Oil-rich biomass (vegetable oil, fats) | Chemical reaction with alcohol | Biodiesel (ready-to-use) |
Ready to select the right biomass conversion technology for your lab or pilot project?
KINTEK specializes in lab equipment and consumables for advanced biofuel research and development. Whether you are exploring pyrolysis, HTL, or transesterification, our precision reactors, heating systems, and analytical tools are designed to support your process development and optimization.
We provide the reliable equipment you need to efficiently convert your specific biomass feedstock into valuable bio-oil. Contact our experts today to discuss your application and find the perfect solution for your laboratory's needs.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- How is a vacuum drying oven utilized in the study of sludge? Preserving Integrity for Precision Analysis
- What materials are used in welding brazing? A Guide to Filler Metals, Fluxes, and Shielding Gases
- How is cannabis distillate extracted? A Step-by-Step Guide to Ultra-Pure THC & CBD
- What are the different deposition techniques? A Guide to PVD, CVD, ALD, and More
- What are the functions of laboratory shakers and centrifuges in phosphorus extraction? Optimize Sample Purification
- Is it safe to store samples at -70°C? A Proven Standard for Long-Term Sample Integrity
- How does sample size affect analysis? Maximize the Reliability of Your Research
- How does an incubator shaker affect the yield of reducing sugars? Optimize Pennisetum alopecuroides Hydrolysis



















