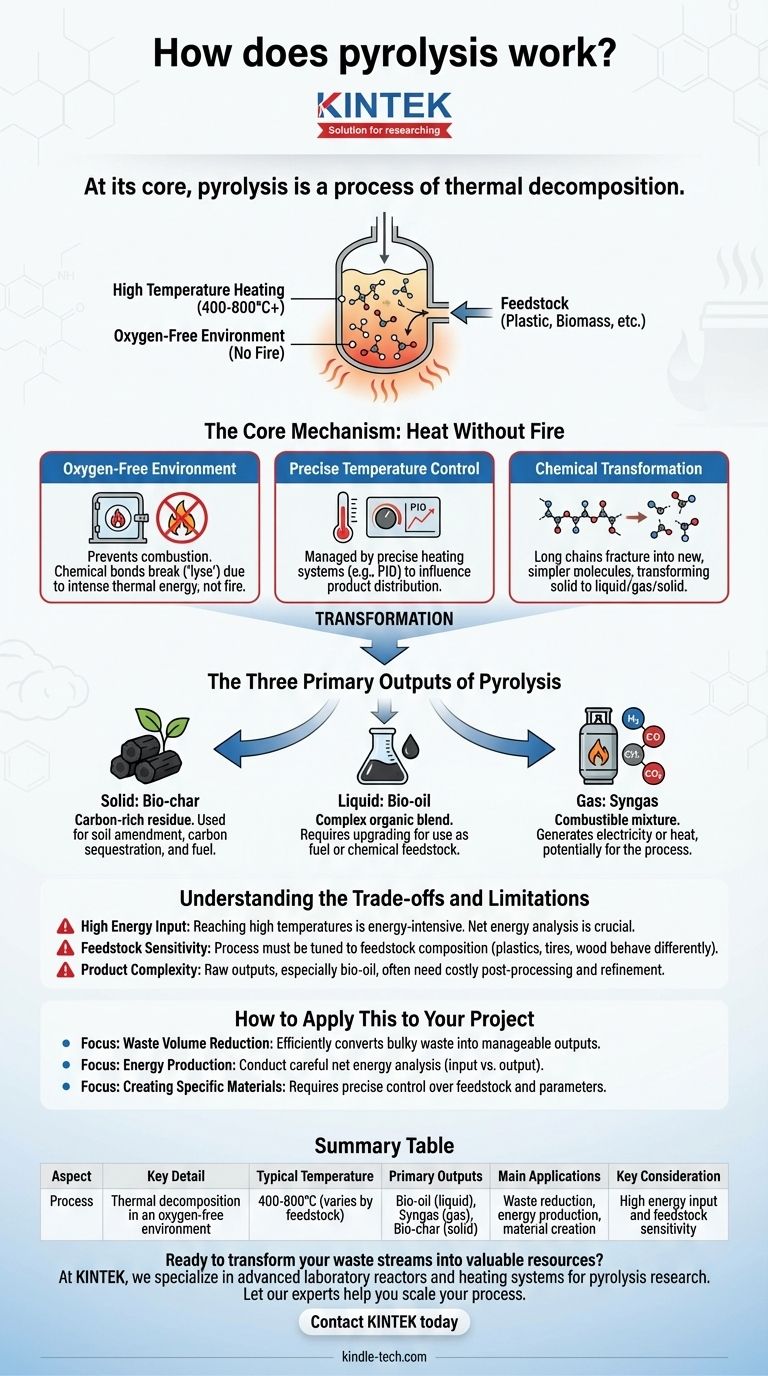
At its core, pyrolysis is a process of thermal decomposition. It involves heating a material, such as plastic or biomass, to a high temperature in an environment completely devoid of oxygen. Instead of burning, the intense heat breaks down the material's complex chemical structure into simpler, smaller molecules, transforming a single input into a mix of gas, liquid, and solid products.
Pyrolysis should not be viewed as simple destruction, but as a controlled transformation. It is a thermochemical tool that deconstructs a feedstock into three distinct and potentially valuable product streams: a combustible gas, a liquid bio-oil, and a solid bio-char.

The Core Mechanism: Heat Without Fire
Pyrolysis is often misunderstood as a form of incineration, but its fundamental principle is the exact opposite. Where burning (combustion) requires oxygen, pyrolysis requires its absence.
The Role of an Oxygen-Free Environment
By removing oxygen, you prevent the material from catching fire. This is the most critical condition for pyrolysis.
Instead of combusting and releasing energy primarily as heat, the chemical bonds within the material are forced to break apart, or "lyse" (from the Greek lysis, meaning separation), due to the intense thermal energy alone.
Precise Temperature Control
The process is managed within a reactor using a precise heating system, often with PID (Proportional-Integral-Derivative) controllers.
Temperature is the primary lever an operator can pull to influence the final product distribution. Different temperatures favor the creation of either more char, more oil, or more gas from the same starting material. Sensitive sensors monitor these conditions to ensure a consistent output.
The Chemical Transformation
The feedstock, typically made of long, complex polymer chains, becomes unstable at high temperatures. The heat causes these chains to fracture into a variety of smaller, less complex molecules.
The result is a new set of chemical products that were not present in the original material. This is why you can turn a solid plastic bottle into a liquid fuel and a combustible gas.
The Three Primary Outputs of Pyrolysis
The specific yield of each output depends heavily on the feedstock and process conditions, but pyrolysis consistently produces three distinct product types.
Solid: Bio-char
This solid, carbon-rich residue is similar to charcoal. It is what remains of the original material after the volatile components have been driven off.
Historically, this was the primary goal of pyrolysis, used to produce charcoal from wood for fuel. Today, bio-char is also valued as a soil amendment and for carbon sequestration.
Liquid: Bio-oil
Also known as pyrolysis oil or tar, this is a complex blend of many different organic compounds. It is produced when the hot gases from the reaction are rapidly cooled and condensed.
This liquid fraction often requires further processing, known as upgrading, to remove oxygen or nitrogen. This step improves its stability and makes it more suitable for use as a renewable fuel or chemical feedstock.
Gas: Syngas
The non-condensable fraction is a mixture of gases, often called syngas (synthesis gas).
This gas typically contains hydrogen, carbon monoxide, carbon dioxide, and methane. It is combustible and can be used to generate electricity or heat, often to help power the pyrolysis process itself, improving its overall energy efficiency.
Understanding the Trade-offs and Limitations
While powerful, pyrolysis is not a silver bullet. Understanding its operational challenges is critical for any practical application.
High Energy Input
The process is energy-intensive. Reaching and maintaining the high temperatures required (often 400-800°C or higher) consumes a significant amount of energy.
The viability of a pyrolysis project often depends on whether the energy value of the products outweighs the energy required to run the system.
Feedstock Sensitivity
Pyrolysis reactors are not "one size fits all." The process must be carefully tuned for different types of feedstock.
Plastics, tires, and wood all have different chemical compositions and will behave differently in the reactor, affecting the ideal temperature, processing time, and ultimately, the product yields.
Product Complexity
The raw outputs, particularly the bio-oil, are often complex mixtures that are not "drop-in" replacements for conventional products. They usually require costly post-processing and refinement before they can be sold or used as high-grade fuel or specialty chemicals.
How to Apply This to Your Project
When evaluating pyrolysis, align the technology's capabilities with your primary strategic goal.
- If your primary focus is waste volume reduction: Pyrolysis is exceptionally effective, converting bulky solid waste like plastics or tires into denser, more manageable, and potentially valuable outputs.
- If your primary focus is energy production: You must conduct a careful net energy analysis, accounting for the energy needed to run the reactor and upgrade the fuels versus the energy content of the final products.
- If your primary focus is creating specific materials: Success depends on precise control over both feedstock quality and reactor parameters to maximize the yield of either char, oil, or gas.
Ultimately, pyrolysis empowers us to reframe waste not as an endpoint, but as a feedstock for creating new value.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process | Thermal decomposition in an oxygen-free environment |
| Typical Temperature | 400-800°C (varies by feedstock) |
| Primary Outputs | Bio-oil (liquid), Syngas (gas), Bio-char (solid) |
| Main Applications | Waste reduction, energy production, material creation |
| Key Consideration | High energy input and feedstock sensitivity |
Ready to transform your waste streams into valuable resources? Pyrolysis is a powerful thermochemical tool, but its success depends on precise control and the right equipment. At KINTEK, we specialize in advanced laboratory reactors and heating systems designed for pyrolysis research and process optimization. Whether you're developing new recycling methods, producing bio-fuels, or creating specialty materials, our robust and reliable equipment ensures accurate temperature control and consistent results. Let our experts help you scale your process from lab to pilot. Contact KINTEK today to discuss how our solutions can power your pyrolysis project and turn your feedstock into profit.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is a high-temperature furnace with multi-probe testing used for ABO3 perovskite? Get Precise Conductivity Data
- What is the function of a high-temperature furnace during burnout? Master Aluminum Foam Production with Precision
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- How do tube furnaces or muffle furnaces ensure stoichiometric accuracy during synthesis? Mastering Li4GeO4 & Li4VO4
- At what temperature does wood pyrolysis begin? Control the Process for Biochar, Bio-Oil, or Syngas



















