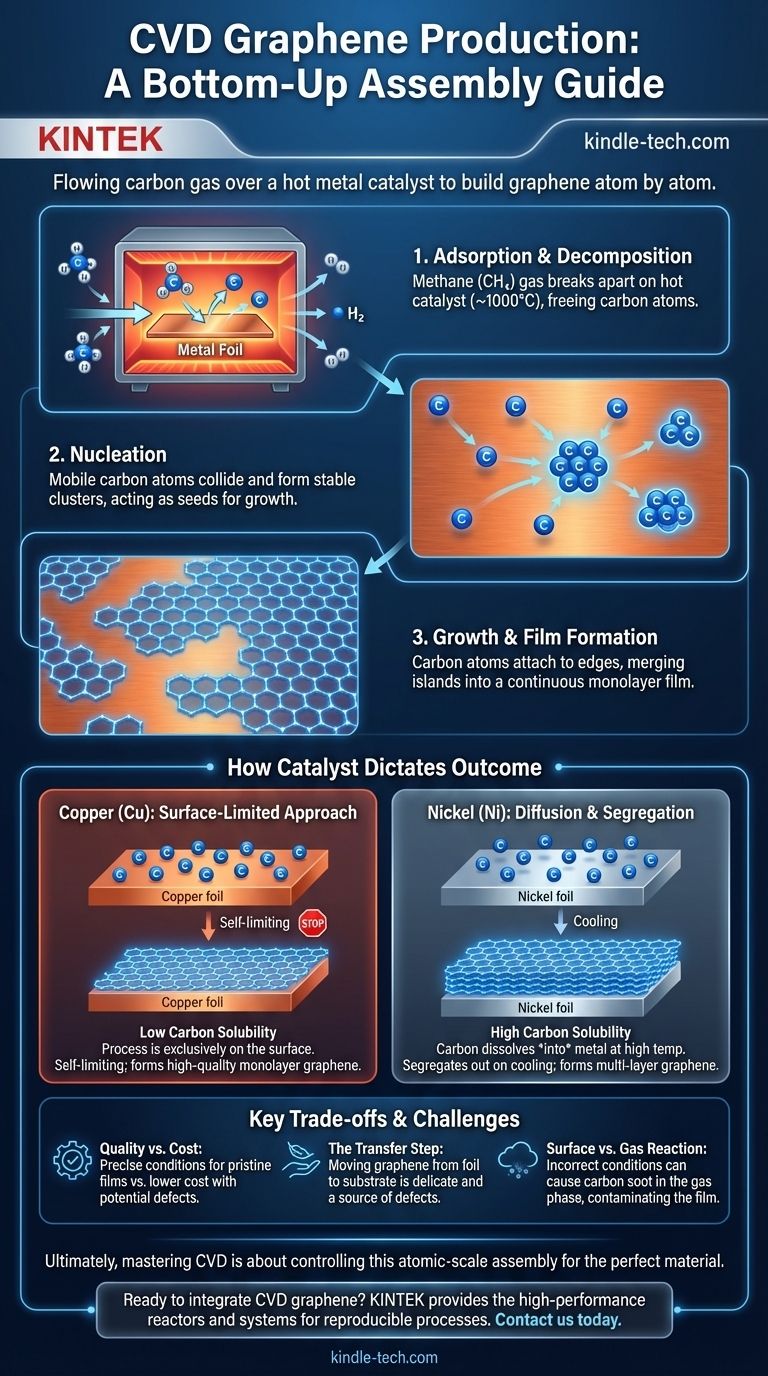
At its core, Chemical Vapor Deposition (CVD) produces graphene by flowing a carbon-containing gas, like methane, over a hot metal catalyst. Inside a high-temperature furnace, the gas decomposes, and the liberated carbon atoms arrange themselves on the surface of the metal foil—typically copper or nickel—into a continuous, single-atom-thick sheet of graphene. Once grown, this graphene film can be transferred to a different substrate for use in electronics or other applications.
Chemical Vapor Deposition is fundamentally a "bottom-up" assembly method. It leverages a catalyst and controlled conditions to precisely build large, high-quality graphene sheets atom by atom, making it the most promising technique for industrial-scale production.

The Core Mechanism of CVD Graphene Growth
Understanding the CVD process is about following the journey of a single carbon atom from a gas molecule to part of a flawless hexagonal lattice. The entire process is a carefully choreographed reaction within a controlled environment.
### The Key Ingredients
The synthesis requires a few essential components: a carbon precursor gas (usually methane), a metal catalyst foil (like copper), carrier gases (hydrogen and argon), and a high-temperature furnace to create the necessary reaction environment.
### Step 1: Adsorption and Decomposition
At temperatures around 1000°C, the precursor gas flows into the reactor. The methane molecules adsorb, or stick, to the surface of the hot metal catalyst. This intense heat causes the methane molecules to break apart, a process known as pyrolysis, freeing carbon atoms on the catalyst's surface.
### Step 2: Nucleation
These newly freed carbon atoms are highly mobile and diffuse across the metal surface. They eventually collide and begin to form small, stable carbon clusters. These clusters act as seeds, or nucleation sites, for graphene growth.
### Step 3: Growth and Film Formation
Once nucleation sites form, other carbon atoms migrating on the surface attach to the edges of these "graphene islands." The islands grow larger and larger until they merge, forming a continuous, unbroken sheet of monolayer graphene that covers the entire surface of the metal foil.
How the Catalyst Dictates the Outcome
The choice of metal catalyst is not arbitrary; it fundamentally changes the growth mechanism and the quality of the resulting graphene. The key difference lies in how well carbon dissolves in the metal.
### Copper: The Surface-Limited Approach
Copper has a very low carbon solubility. This means carbon atoms do not dissolve into the bulk of the copper. Instead, the entire process happens directly and exclusively on the surface.
This is a self-limiting mechanism. Once the copper surface is fully covered by a single layer of graphene, the catalytic activity stops, preventing the formation of additional layers. This makes copper the ideal substrate for producing large areas of high-quality, monolayer graphene.
### Nickel: The Diffusion and Segregation Method
In contrast, nickel has a high carbon solubility. At high temperatures, carbon atoms from the precursor gas dissolve into the bulk of the nickel metal, much like sugar dissolving in hot water.
When the system is cooled, the nickel's ability to hold carbon decreases, and the dissolved carbon atoms "precipitate" or segregate back to the surface, where they form graphene layers. This process is harder to control and often results in thicker, less uniform, or multi-layer graphene.
Understanding the Trade-offs
While CVD is a powerful technique, it is essential to recognize its inherent challenges and limitations. These trade-offs define the landscape of graphene production.
### Quality vs. Cost
Achieving pristine, defect-free graphene requires extremely precise control over temperature, pressure, and gas flow rates, which increases complexity and cost. Relaxing these conditions can lower the cost but may introduce defects or impurities into the graphene sheet.
### The Critical Transfer Step
Graphene grown via CVD is created on a metal foil, which is not useful for most final applications like electronics. The graphene must be transferred to a target substrate, such as silicon or a flexible polymer.
This transfer process is delicate and is a primary source of defects like rips, wrinkles, and contamination, which can degrade the graphene's exceptional properties. The challenge of a clean, scalable transfer remains a significant bottleneck.
### Surface vs. Gas Reaction
For high-quality film, the methane decomposition must occur on the catalyst surface (a heterogeneous reaction). If the temperature is too high or conditions are wrong, carbon can form soot particles in the gas phase, which then fall and contaminate the growing graphene layer, severely degrading its quality.
Making the Right Choice for Your Goal
The optimal CVD approach depends entirely on the intended application and desired properties of the final material.
- If your primary focus is producing large-area, high-quality monolayer graphene for electronics: CVD on a copper substrate is the established standard due to its self-limiting surface growth mechanism.
- If your goal is to produce multi-layer graphene or graphene powders: A diffusion-based method using a nickel catalyst might be a more direct and cost-effective route.
- If you are conducting fundamental research: The precise control offered by CVD makes it an invaluable tool for systematically studying the effects of temperature, precursors, and catalysts on graphene's properties.
Ultimately, mastering CVD for graphene production is about controlling a catalyzed, atomic-scale assembly process to build a perfect material from the bottom up.
Summary Table:
| Stage | Key Process | Role of Catalyst | Outcome |
|---|---|---|---|
| 1. Adsorption & Decomposition | Methane gas flows over hot metal foil (~1000°C) and decomposes. | Provides a hot surface for gas molecules to break apart, releasing carbon atoms. | Carbon atoms are freed on the catalyst surface. |
| 2. Nucleation | Free carbon atoms diffuse and form stable clusters. | The surface properties determine the density and location of nucleation sites. | Small "graphene islands" begin to form. |
| 3. Growth | Carbon atoms attach to the edges of the islands, which expand and merge. | Dictates the growth mechanism (surface-limited vs. diffusion-based). | A continuous, single-atom-thick graphene film is formed. |
Ready to integrate high-quality CVD graphene into your research or product development?
The precise control required for successful graphene synthesis relies on high-performance lab equipment. KINTEK specializes in providing the reactors, furnaces, and gas handling systems that enable reproducible and scalable CVD processes.
Whether you are developing next-generation electronics or conducting cutting-edge materials research, our expertise in lab equipment and consumables can help you achieve your goals. Contact our experts today to discuss how we can support your laboratory's specific needs.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
People Also Ask
- What are the main advantages of Chemical Vapor Deposition (CVD)? Achieve Precision Coating for Complex Geometries
- What function does CVD equipment serve in rhodium-modified coatings? Achieve Deep Diffusion and Microstructural Precision
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis



















