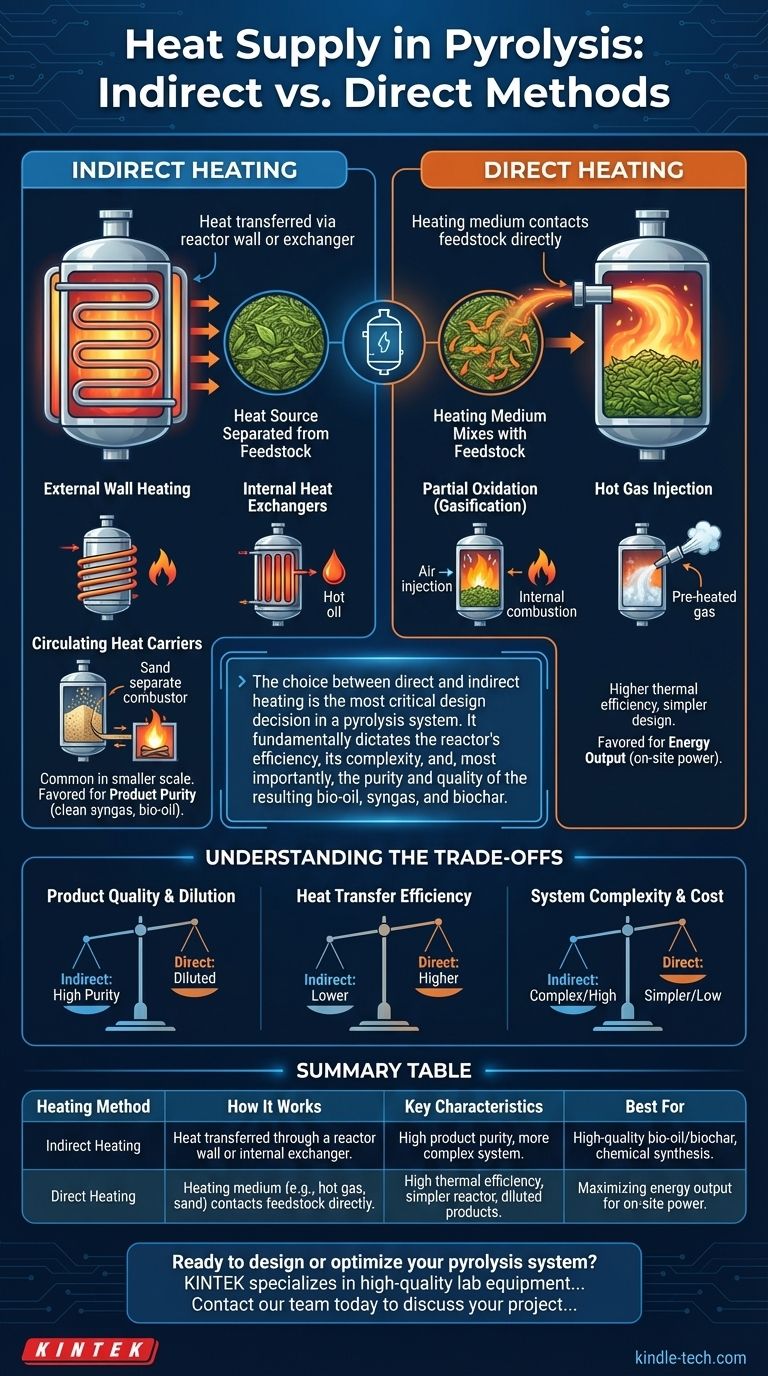
In pyrolysis, heat is supplied through two primary methods: indirect and direct heating. Indirect heating, the more common approach, transfers thermal energy through a physical barrier like a reactor wall, ensuring the heating medium never mixes with the feedstock. Direct heating involves the heating medium making direct contact with the feedstock, which is often simpler but can impact the final product composition.
The choice between direct and indirect heating is the most critical design decision in a pyrolysis system. It fundamentally dictates the reactor's efficiency, its complexity, and, most importantly, the purity and quality of the resulting bio-oil, syngas, and biochar.

Indirect vs. Direct Heating: The Fundamental Divide
Understanding how heat gets to the biomass is the first step in understanding any pyrolysis reactor. The core difference lies in whether the source of heat and the feedstock share the same space.
What is Indirect Heating?
In indirect heating, the energy source (like flue gas from a burner or an electric element) is kept separate from the biomass. Heat must be transferred through a solid medium, most commonly the steel wall of the reactor.
This is analogous to cooking food in a covered pot on a stove. The flame doesn't touch the food; heat conducts through the bottom of the pot.
What is Direct Heating?
In direct heating, the heating medium is introduced directly into the reactor and mixes intimately with the biomass. This allows for very rapid and efficient heat transfer.
Think of this like a steam cooker, where hot steam is injected directly onto the food, or a gas grill where the hot combustion gases flow directly over the food.
Common Indirect Heating Methods
Indirect heating is favored when product purity is the primary goal, as it prevents contamination from combustion gases.
External Wall Heating
This is the simplest method. The reactor vessel is heated from the outside using electric heating elements or by burning fuel in an external jacket or furnace.
This approach is common in smaller-scale or simpler reactors like screw augers and rotary kilns. Its main limitation is poor heat transfer, which makes it difficult to scale up effectively.
Internal Heat Exchangers
To improve heat transfer, heated tubes or plates can be placed inside the reactor. A hot fluid, such as thermal oil or molten salt, flows through these internal components, transferring heat more directly to the biomass bed.
This is a more efficient method than simple external wall heating but adds mechanical complexity to the reactor's design.
Circulating Heat Carriers
This is a highly efficient method used in large-scale fluidized bed reactors. An inert solid material, like sand, is used as a heat carrier.
The sand is heated in a separate combustion chamber and then transported into the pyrolysis reactor. There, it mixes with the biomass, rapidly transferring its heat before being cycled back to the combustor to be reheated. This creates a continuous, highly efficient heat loop.
Common Direct Heating Methods
Direct heating is often chosen for its high thermal efficiency and simpler reactor design, though it comes at the cost of product purity.
Partial Oxidation (Gasification)
In this method, a controlled amount of oxygen (or air) is deliberately introduced into the reactor. This causes a portion of the feedstock or pyrolysis gases to combust.
This internal combustion generates the intense heat needed to pyrolyze the remaining feedstock. While efficient, this process dilutes the final syngas with nitrogen (if air is used) and CO2, reducing its energy density and making it less suitable for chemical synthesis.
Hot Gas Injection
This method involves injecting a pre-heated, non-reactive gas directly into the reactor. Common choices include superheated steam or recycled, reheated syngas from the pyrolysis process itself.
This provides the rapid heat transfer of a direct method without diluting the product with combustion byproducts like CO2. However, it requires a separate, large-scale system to heat the gas before injection.
Understanding the Trade-offs
No single heating method is universally superior. The optimal choice depends on balancing product quality, efficiency, and cost.
Product Quality and Dilution
Indirect heating produces a "clean" syngas and bio-oil, free from combustion byproducts. This is critical if the products are intended for upgrading into high-value chemicals or transportation fuels.
Direct heating via partial oxidation always results in a diluted syngas, which is typically better suited for immediate, on-site heat and power generation rather than for synthesis.
Heat Transfer Efficiency
Direct contact methods (like partial oxidation or using a circulating heat carrier) offer significantly higher rates of heat transfer than heating through a reactor wall.
This efficiency is crucial for fast pyrolysis, where biomass must be heated to reaction temperature in seconds to maximize liquid bio-oil yield.
System Complexity and Cost
Externally heated screw reactors are mechanically simple and relatively low-cost, making them suitable for smaller, distributed applications.
In contrast, a dual fluidized bed system with a circulating heat carrier is a complex, capital-intensive installation suited for large, industrial-scale processing where efficiency is paramount.
Making the Right Choice for Your Goal
The heating method must be selected based on the desired end-product and operational scale.
- If your primary focus is high-quality, undiluted bio-oil or biochar: Your best choice is an indirect heating method, such as a circulating fluid bed or an externally heated auger reactor.
- If your primary focus is maximizing energy output for on-site power: Direct heating via partial oxidation offers a simpler, thermally self-sufficient system, even if the syngas is diluted.
- If your primary focus is large-scale, high-throughput processing: A circulating fluid bed reactor using an inert heat carrier (indirect heating) provides the unmatched heat transfer required for industrial capacity.
Ultimately, the method of heat supply is not just a component; it is a core design principle that defines the capabilities and limitations of the entire pyrolysis system.
Summary Table:
| Heating Method | How It Works | Key Characteristics | Best For |
|---|---|---|---|
| Indirect Heating | Heat transferred through a reactor wall or internal exchanger. | High product purity, more complex system. | High-quality bio-oil/biochar, chemical synthesis. |
| Direct Heating | Heating medium (e.g., hot gas, sand) contacts feedstock directly. | High thermal efficiency, simpler reactor, diluted products. | Maximizing energy output for on-site power. |
Ready to design or optimize your pyrolysis system? The choice of heating method is critical to achieving your target product yields and purity. KINTEK specializes in high-quality lab equipment and consumables for pyrolysis R&D and process development. Our experts can help you select the right technology for your specific biomass and goals. Contact our team today to discuss your project and ensure optimal thermal performance.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















