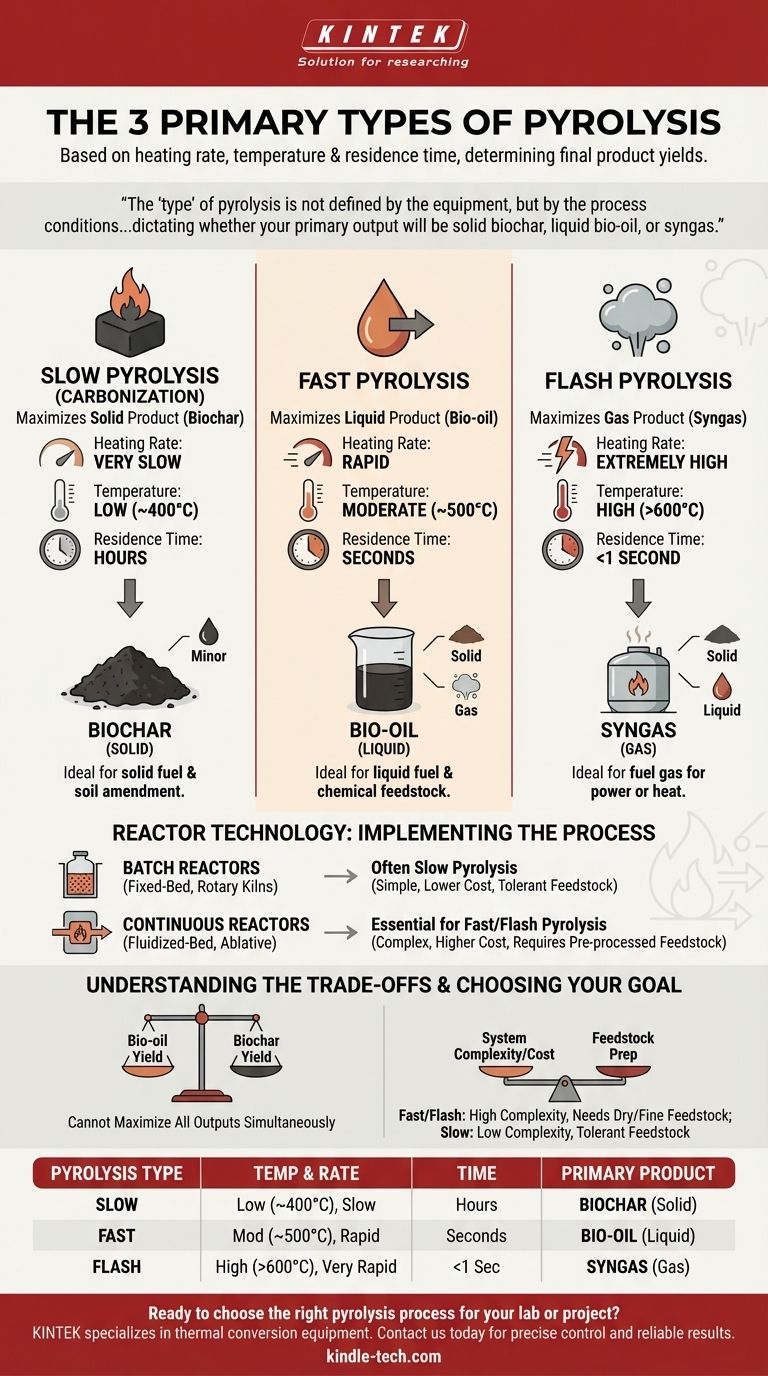
In practice, pyrolysis is categorized into three primary types based on the heating rate and residence time of the material. While there are many different reactor designs, these three process classifications—slow, fast, and flash—are the most critical distinction, as they directly determine the final product yields of solid char, liquid bio-oil, and combustible gas.
The "type" of pyrolysis is not defined by the equipment, but by the process conditions. The fundamental choice is between slow, fast, and flash pyrolysis, which dictates whether your primary output will be solid biochar, liquid bio-oil, or syngas. The reactor technology is then selected to achieve those specific conditions.

The Core Differentiator: Process Speed and Temperature
The most important way to classify pyrolysis is by the speed at which biomass is heated and the time it spends at peak temperature. This determines the chemical reaction pathways and, therefore, the composition of the final products.
Slow Pyrolysis (Carbonization)
Slow pyrolysis involves heating organic material at a low temperature (around 400°C) over a long period, often hours. The heating rate is very slow.
This process is optimized to produce the maximum amount of solid product, known as biochar or charcoal. It produces relatively small amounts of liquid and gas byproducts.
Fast Pyrolysis
Fast pyrolysis uses a much faster heating rate to bring the material to a moderate temperature (around 500°C) in just a few seconds. The material is then cooled quickly.
This process is designed to maximize the yield of liquid product, known as bio-oil. The rapid heating and short reaction time "freeze" the intermediate decomposition products before they can break down further into gas and char.
Flash Pyrolysis
Flash pyrolysis represents the most extreme conditions, with extremely high heating rates and very high temperatures (often above 600°C). The residence time is typically less than a second.
This method is primarily used to maximize the production of non-condensable gases (syngas), which is a mixture of hydrogen, carbon monoxide, carbon dioxide, and methane. This gas can be used directly as fuel.
How Pyrolysis is Implemented: Reactor Technology
The choice of reactor or furnace is a practical decision made to achieve the conditions required for slow, fast, or flash pyrolysis. The long list of reactor types reflects different engineering solutions to control heat transfer and material flow.
Classifying by Operational Mode
The simplest classification is based on how material is fed into the system.
- Batch Reactors: These are loaded with a set amount of feedstock, sealed, and run through a complete heating cycle. They are common for small-scale applications and are typical for slow pyrolysis (e.g., traditional charcoal kilns).
- Continuous Reactors: These are fed with a constant stream of feedstock and continuously discharge product. They are essential for large-scale industrial applications and are required for the precise control needed in fast and flash pyrolysis.
Common Reactor Designs
Different reactor designs are optimized for specific types of heat transfer.
- Fixed-Bed Reactors: In these, the biomass remains in a static pile or "bed" as hot gases pass through it. This design provides poor heat transfer and is generally only suitable for slow pyrolysis.
- Fluidized-Bed Reactors: A hot gas is forced through a bed of fine particles (like sand) at high velocity, causing it to behave like a fluid. When biomass is introduced, it is mixed rapidly and heated almost instantly, making this design ideal for fast pyrolysis.
- Rotary Kilns: A large, rotating cylinder is heated from the outside. The rotation tumbles and mixes the material, providing more uniform heating than a fixed bed. These can be adapted for slow or intermediate pyrolysis.
Understanding the Trade-offs
Selecting a pyrolysis type involves balancing competing factors. No single method is universally superior; the optimal choice depends entirely on your goal and feedstock.
The Product Yield Dilemma
You cannot simultaneously maximize all outputs. The conditions that favor bio-oil production (fast heating, short residence time) inherently suppress the formation of biochar.
Conversely, the long residence times needed to create high-quality, stable biochar will crack many of the valuable vapors that would have formed bio-oil, turning them into less valuable gas.
Feedstock and Preparation
Fast and flash pyrolysis require very small, dry particles to enable rapid heat transfer. This often means significant energy and cost must go into drying and grinding the raw feedstock before it even enters the reactor.
Slow pyrolysis is far more tolerant of larger, wetter feedstock, reducing the need for extensive pre-processing.
System Complexity and Cost
The equipment required for fast and flash pyrolysis (e.g., fluidized-bed or ablative reactors) is mechanically complex and expensive to build and operate. These systems are only economical at a large, continuous industrial scale.
Slow pyrolysis systems, especially batch kilns, can be very simple and relatively inexpensive, making them accessible for smaller-scale or distributed operations.
Making the Right Choice for Your Goal
The best pyrolysis method is the one that produces the output you value most. The process conditions are your primary lever for controlling this outcome.
- If your primary focus is solid fuel or soil amendment: You should use slow pyrolysis to maximize the yield and quality of biochar.
- If your primary focus is liquid fuel or chemical feedstock: You should use fast pyrolysis to maximize the production of bio-oil.
- If your primary focus is generating fuel gas for power or heat: You should use flash or high-temperature gasification (a related process) to maximize syngas output.
Understanding these core classifications empowers you to select the precise thermal conversion pathway that aligns with your specific material and desired outcome.
Summary Table:
| Pyrolysis Type | Heating Rate & Temperature | Residence Time | Primary Product |
|---|---|---|---|
| Slow Pyrolysis | Low (~400°C), slow heating | Hours | Biochar (Solid) |
| Fast Pyrolysis | Moderate (~500°C), rapid heating | Seconds | Bio-oil (Liquid) |
| Flash Pyrolysis | High (>600°C), very rapid heating | <1 Second | Syngas (Gas) |
Ready to choose the right pyrolysis process for your lab or project? KINTEK specializes in providing high-quality lab equipment and consumables tailored to your thermal conversion needs. Whether you're optimizing for biochar, bio-oil, or syngas production, our expertise ensures precise control and reliable results. Contact us today to discuss how we can support your laboratory's pyrolysis applications!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Mesh belt controlled atmosphere furnace
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process



















