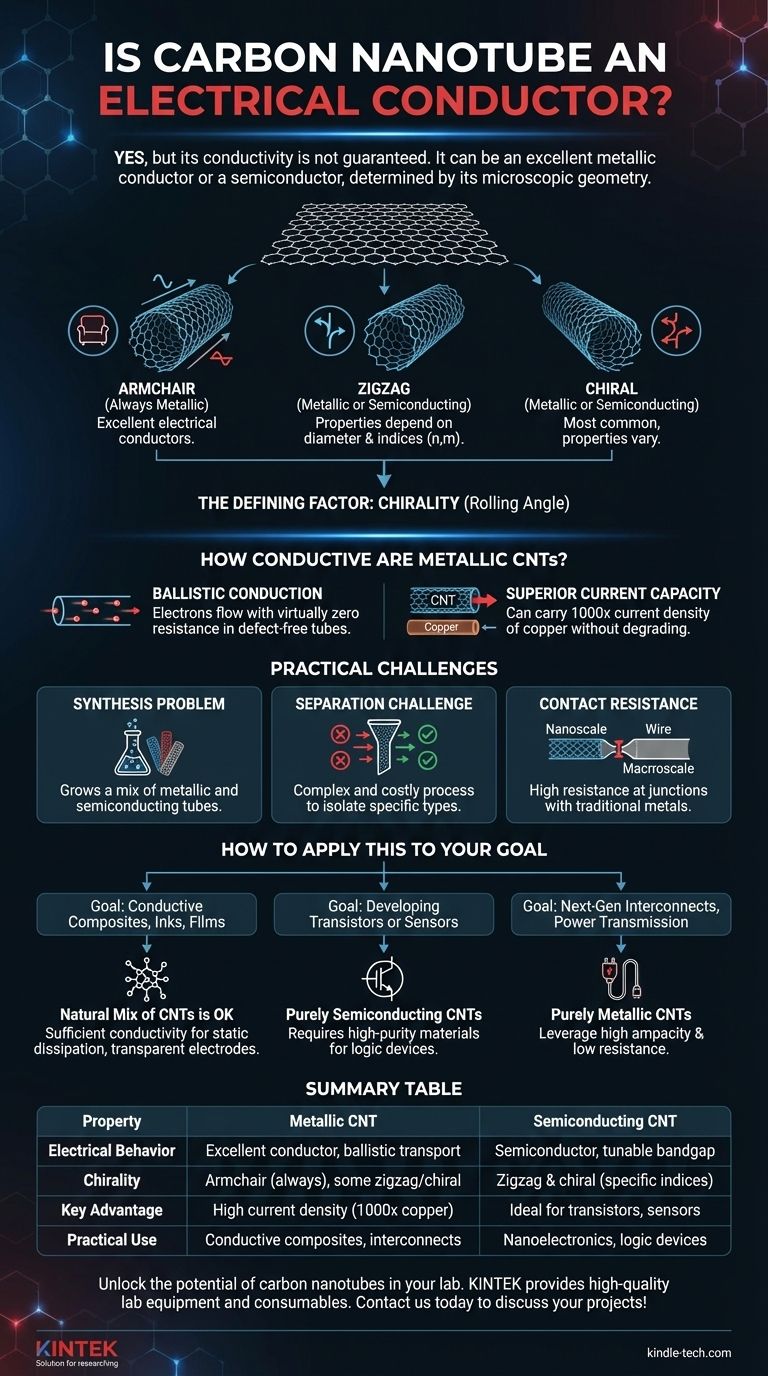
Yes, but its conductivity is not guaranteed. A carbon nanotube (CNT) can be an excellent metallic conductor, sometimes outperforming copper, or it can be a semiconductor. This dual nature is one of its most fascinating and challenging properties, and it is determined entirely by the tube's microscopic geometry.
The core principle to understand is that a carbon nanotube's electrical behavior is not fixed. It is dictated by its chirality—the specific angle at which the foundational graphene sheet is "rolled" to form the tube's cylindrical structure.
The Defining Factor: Atomic Structure
To understand why a CNT's conductivity varies, we must look at how it's made at the atomic level. This reveals why geometry is everything.
Graphene as the Foundation
Imagine a carbon nanotube as a single sheet of graphene—a one-atom-thick layer of carbon atoms in a honeycomb lattice—that has been seamlessly rolled into a cylinder. The electrical properties of that original graphene sheet are inherited by the tube.
What is Chirality?
Chirality refers to the angle and direction in which the graphene sheet is rolled. Think of rolling a piece of paper with a honeycomb pattern on it. You can roll it straight, at a slight angle, or at a sharp angle.
Each of these rolling methods results in a different alignment of the honeycomb pattern along the tube's axis, fundamentally altering how electrons can move through it.
Armchair, Zigzag, and Chiral Tubes
This "rolling" angle determines the final structure, which falls into three main categories:
- Armchair: When rolled in a specific way, the resulting pattern at the tube's opening resembles a row of armchairs. Armchair CNTs are always metallic and behave as excellent electrical conductors.
- Zigzag: This structure is formed by rolling the sheet in another specific orientation. These tubes can be either metallic or semiconducting.
- Chiral: These are tubes rolled at any other angle between the armchair and zigzag configurations. The vast majority of CNTs are chiral, and they can also be either metallic or semiconducting.
Whether a zigzag or chiral tube is metallic or semiconducting depends on its precise diameter and chiral angle, a relationship defined by specific mathematical indices (n,m).
How Conductive Are Metallic CNTs?
When a carbon nanotube is metallic, its performance can be extraordinary, far surpassing traditional conductors in key areas.
Ballistic Conduction
Under certain conditions, electrons can pass through short, defect-free CNTs without scattering or bumping into atoms. This phenomenon, known as ballistic conduction, means they flow with virtually zero resistance.
Superior Current Capacity
A key advantage of CNTs is their ability to carry immense electrical current densities—more than 1,000 times that of copper—without degrading. This property, known as ampacity, makes them highly attractive for future microelectronics where components are densely packed.
Understanding the Practical Challenges
While the theoretical properties of CNTs are remarkable, their practical application in electronics faces significant hurdles related to their structural dependency.
The Synthesis Problem
Current manufacturing methods, such as chemical vapor deposition, typically produce a mixture of CNTs with different chiralities. This means any batch of as-grown CNTs will contain a mix of metallic and semiconducting tubes.
The Separation Challenge
For most electronic applications, a pure sample is required. Using a mix of CNTs to create a transistor, for example, would result in faulty devices, as the metallic tubes would create short circuits.
Separating metallic from semiconducting CNTs is a complex and costly process that remains a primary obstacle to their widespread adoption in semiconductor manufacturing.
Contact Resistance
Effectively connecting a nanoscale tube to a macroscale metal wire or electrode is not trivial. High contact resistance can form at this junction, creating a bottleneck that negates the benefits of the CNT's low internal resistance.
How to Apply This to Your Goal
Your approach to using carbon nanotubes depends entirely on whether their variable conductivity is a benefit or a hindrance for your specific purpose.
- If your primary focus is creating conductive composites, inks, or films: The natural mix of CNTs is often acceptable. The metallic tubes (typically one-third of the batch) will form a percolating network that provides sufficient conductivity for applications like electrostatic dissipation or transparent electrodes.
- If your primary focus is developing transistors or sensors: You require purely semiconducting CNTs. The main challenge is sourcing these high-purity materials or implementing effective post-synthesis separation techniques.
- If your primary focus is next-generation interconnects or power transmission: You require purely metallic CNTs to leverage their high ampacity and low resistance. The goal would be to develop synthesis methods that exclusively grow armchair tubes or to find a scalable way to isolate them.
Ultimately, a carbon nanotube is a material whose immense potential is unlocked only when its specific atomic structure is precisely controlled.
Summary Table:
| Property | Metallic CNT | Semiconducting CNT |
|---|---|---|
| Electrical Behavior | Excellent conductor, ballistic transport | Semiconductor, tunable bandgap |
| Chirality | Armchair (always metallic), some zigzag/chiral | Zigzag and chiral tubes (specific indices) |
| Key Advantage | High current density (1000x copper), low resistance | Ideal for transistors, sensors |
| Practical Use | Conductive composites, interconnects | Nanoelectronics, logic devices |
Unlock the potential of carbon nanotubes in your lab. Whether you need conductive materials for composites or semiconducting tubes for advanced electronics, KINTEK provides high-quality lab equipment and consumables tailored to your research. Our expertise supports precise material synthesis and characterization—contact us today to discuss how we can enhance your nanotechnology projects!
Visual Guide
Related Products
- Large Vertical Graphite Vacuum Graphitization Furnace
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Hexagonal Boron Nitride HBN Ceramic Ring
- Platinum Auxiliary Electrode for Laboratory Use
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
People Also Ask
- What is the thermal property of graphite? Mastering Extreme Heat Management
- Is high ash content good? A Guide to Understanding Pet Food Mineral Levels
- What role does a laboratory high-temperature furnace play in PHT? Engineer Nano-Scale Coating Durability
- What role do high-temperature furnaces play in SOFC synthesis? Precision Tools for Electrolyte & Electrode Optimization
- How does a high-temperature furnace contribute to the post-synthesis heat treatment of Fe-Cr-Mn-Mo-N-C composites?



















