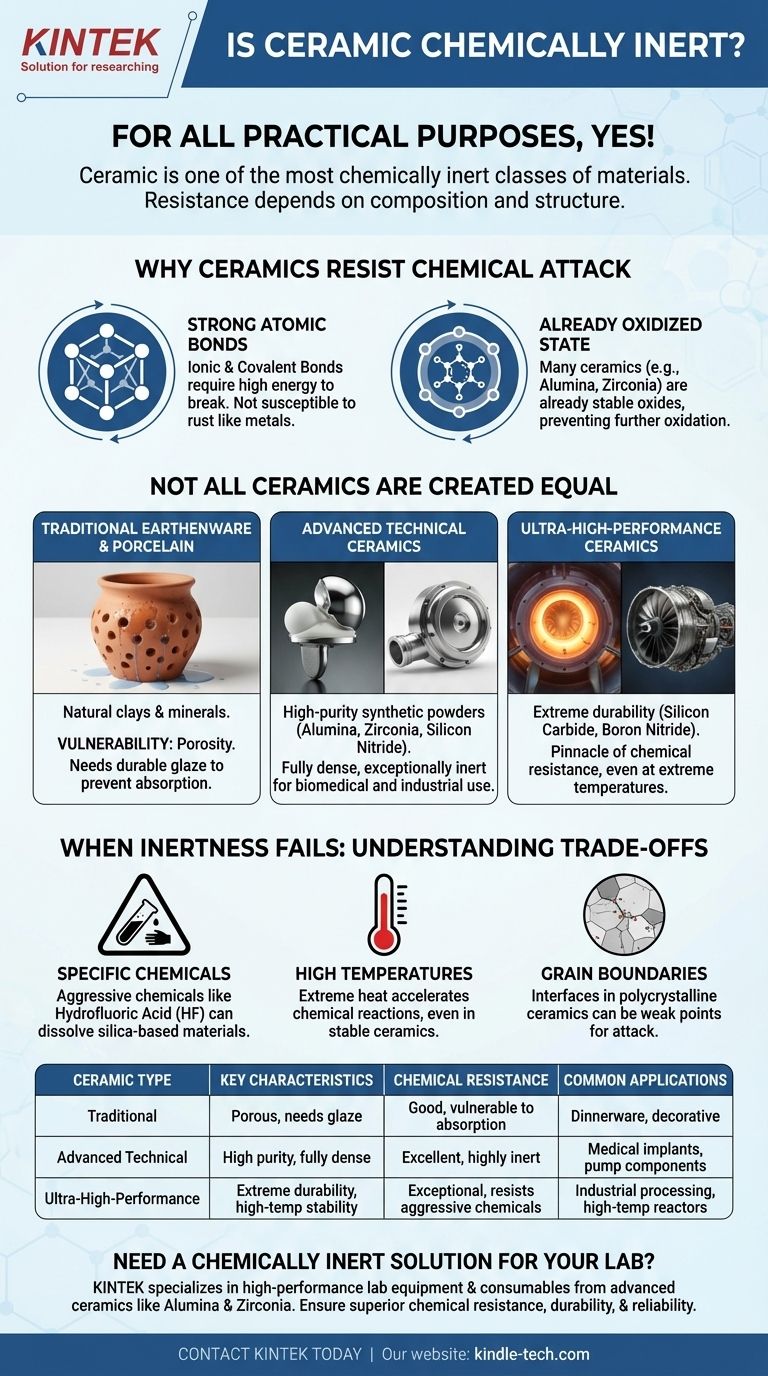
For all practical purposes, yes, ceramic is one of the most chemically inert classes of materials available. This exceptional resistance to chemical attack is a primary reason it's used in everything from medical implants to industrial chemical processing. However, "ceramic" is a vast category, and its level of inertness depends entirely on its specific chemical composition and physical structure.
While no material is perfectly inert under all conditions, advanced technical ceramics come exceptionally close. Their resistance stems from powerful atomic bonds and an already-oxidized state, but it is crucial to distinguish them from traditional ceramics and to match the specific ceramic grade to the chemical environment.

Why Ceramics Resist Chemical Attack
The remarkable stability of ceramic materials is not an accident; it is a direct result of their fundamental atomic structure. Understanding this provides a clear framework for evaluating their performance.
The Power of Strong Atomic Bonds
Ceramic materials are characterized by extremely strong ionic and covalent bonds. These bonds hold atoms together in a rigid, stable lattice, requiring a significant amount of energy to break.
Unlike metals, which have a "sea" of mobile electrons that make them susceptible to electrochemical reactions like rust, the electrons in ceramics are tightly held. This makes it very difficult for chemical agents to disrupt the structure and cause corrosion.
An Already Oxidized State
Many high-performance ceramics, such as alumina (aluminum oxide) and zirconia (zirconium dioxide), are already oxides. In simple terms, they are already in their most stable, low-energy state—they cannot be further oxidized or "rusted."
This inherent stability means they do not readily react with their environment to form new compounds, a core principle of their chemical inertness.
Not All Ceramics Are Created Equal
The term "ceramic" covers everything from a simple terracotta pot to a high-purity component inside a jet engine. Their chemical resistance varies significantly.
Traditional Earthenware and Porcelain
These ceramics are typically made from natural clays (like kaolinite) and minerals (like silica and feldspar). While generally very resistant to common acids, bases, and solvents, their primary vulnerability can be porosity.
If not fully vitrified or protected by a durable, non-porous glaze, these materials can absorb liquids. This can lead to physical degradation or allow trapped chemicals to react slowly over time.
Advanced Technical Ceramics
This category includes materials engineered for extreme performance, such as alumina, zirconia, and silicon nitride. They are manufactured from ultra-pure synthetic powders and sintered at high temperatures to achieve near-total density.
Their high purity and lack of porosity make them exceptionally inert across a wide range of corrosive environments. This is why they are the material of choice for demanding applications like biomedical implants, pump components, and chemical reactor linings.
Ultra-High-Performance Ceramics
Materials like silicon carbide (SiC) and boron nitride (BN) represent the pinnacle of chemical resistance, especially at extreme temperatures. They can withstand some of the most aggressive chemical environments where even high-grade metals and other ceramics would fail.
Understanding the Trade-offs: When Inertness Fails
Despite their robust nature, ceramics are not invincible. Acknowledging their limitations is critical for correct material selection.
The Threat of Specific Chemicals
Certain aggressive chemicals can attack specific types of ceramics. The classic example is hydrofluoric acid (HF), which is notable for its ability to dissolve silica-based materials, including glass and some traditional ceramics.
Similarly, very strong hot alkali (basic) solutions can slowly etch the surface of some ceramic types over long periods.
High Temperatures as a Catalyst
While many ceramics are valued for their high-temperature stability, extreme heat always accelerates the rate of chemical reactions. A ceramic that is perfectly inert at room temperature might show slight reactivity when exposed to the same chemical at 1000°C.
The Weakness of Grain Boundaries
In a polycrystalline ceramic, the interfaces between the individual crystal grains, known as grain boundaries, can be points of weakness. Impurities can accumulate here, creating sites that are more susceptible to chemical attack than the bulk crystal itself. This is why purity and processing are critical in advanced ceramics.
Making the Right Choice for Your Application
Your choice of ceramic must be driven by the specific demands of your environment and performance requirements.
- If your primary focus is biocompatibility for medical devices: Choose high-purity, fully dense technical ceramics like zirconia or medical-grade alumina, which are proven to be non-toxic and non-reactive with bodily fluids.
- If your primary focus is containing highly corrosive chemicals in industry: Look to specialized technical ceramics like silicon carbide or high-purity alumina, and always verify their resistance against your specific chemical concentrations and operating temperatures.
- If your primary focus is everyday use like cookware or dinnerware: High-quality, non-porous porcelain or ceramics with a durable, impermeable glaze are perfectly safe, non-leaching, and non-reactive for all food applications.
By understanding these critical distinctions, you can confidently leverage the remarkable chemical stability of the correct ceramic for your specific goal.
Summary Table:
| Ceramic Type | Key Characteristics | Chemical Resistance | Common Applications |
|---|---|---|---|
| Traditional (Earthenware) | Porous, may require a glaze | Good, but vulnerable to absorption | Dinnerware, decorative items |
| Advanced Technical (Alumina, Zirconia) | High purity, fully dense | Excellent, highly inert | Medical implants, pump components |
| Ultra-High-Performance (Silicon Carbide) | Extreme durability, high-temperature stability | Exceptional, resists aggressive chemicals | Industrial chemical processing, high-temperature reactors |
Need a chemically inert solution for your lab?
Selecting the right ceramic material is critical for the success and safety of your laboratory processes. KINTEK specializes in high-performance lab equipment and consumables, including components made from advanced technical ceramics like alumina and zirconia. Our expertise ensures you get materials that offer superior chemical resistance, durability, and reliability for your specific application—from handling corrosive chemicals to biomedical research.
Let us help you enhance your lab's capabilities and safety. Contact our experts today to discuss your requirements and discover the perfect ceramic solution from KINTEK.
Visual Guide
Related Products
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Precision Machined Silicon Nitride (SiN) Ceramic Sheet for Engineering Advanced Fine Ceramics
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- Hexagonal Boron Nitride HBN Ceramic Ring
People Also Ask
- Which of the following is used in furnace to withstand high temperature? Key Materials for Extreme Heat
- What functions do high-purity alumina support rods serve in sCO2 experiments? Ensure High-Temp Material Integrity
- What is the maximum operating temperature of alumina? The Critical Role of Purity and Form
- Why are high-purity alumina rods used in LOCA experiments? Simulating Nuclear Fuel Gap and Steam Starvation
- Why are ceramics more resistant to corrosion? Unlock the Secret to Unmatched Chemical Stability



















