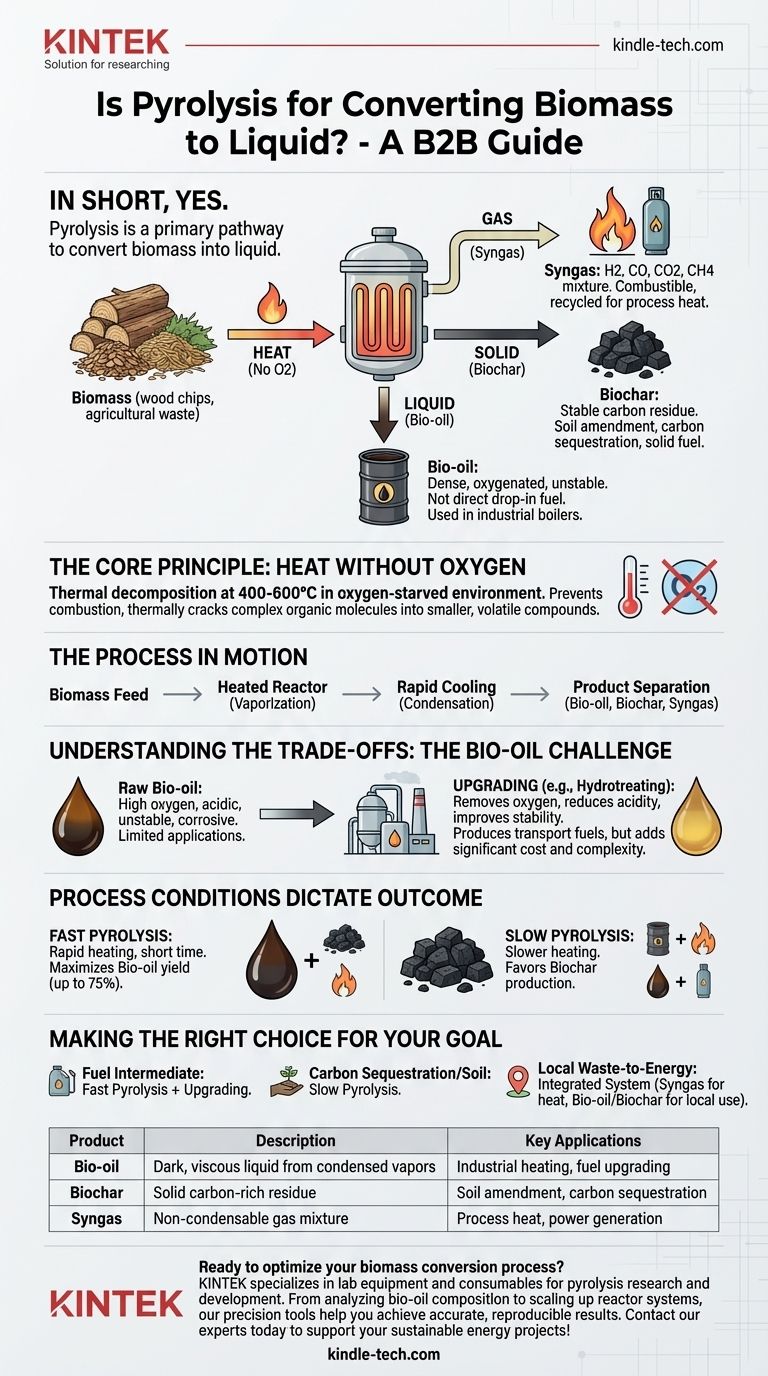
In short, yes. Pyrolysis is a primary thermochemical pathway specifically used to convert biomass into a liquid product. This process breaks down organic materials with heat in the absence of oxygen, producing not only a liquid known as bio-oil but also a solid (biochar) and a gas (syngas).
The core function of pyrolysis is to deconstruct complex biomass into a portfolio of simpler products. While it effectively produces a liquid, the "usefulness" of that liquid depends entirely on the intended application, as it is chemically very different from conventional petroleum and often requires further processing.

What is Pyrolysis and How Does It Work?
Pyrolysis is a foundational technology in the field of biomass conversion. Understanding its core mechanism is key to appreciating its potential and limitations.
The Core Principle: Heat Without Oxygen
Pyrolysis is the thermal decomposition of organic material at high temperatures (typically 400-600°C) in an oxygen-starved environment.
Crucially, the lack of oxygen prevents combustion (burning). Instead of burning away, the large organic molecules that make up biomass—like cellulose and lignin—are thermally cracked into smaller, volatile compounds.
The Process in Motion
A pyrolysis plant feeds biomass into a heated reactor. As the material heats up, it breaks down and vaporizes. These hot vapors are then rapidly cooled, causing them to condense into the liquid product, while non-condensable gases and a solid carbon residue are separated.
The Three Key Products of Biomass Pyrolysis
The term "conversion" is important because pyrolysis doesn't just create one product. It fractionates the biomass into three distinct streams, each with its own value.
1. Bio-oil (The Liquid)
This is the primary liquid product, often called pyrolysis oil. It is a dark, dense, and viscous liquid that represents a significant portion of the original biomass energy.
However, bio-oil is a complex mixture of hundreds of oxygenated organic compounds. It is acidic, contains significant water, and is chemically unstable, meaning it is not a direct "drop-in" replacement for diesel or gasoline.
2. Biochar (The Solid)
After the volatile components have been driven off, a solid, carbon-rich material called biochar remains. This is the "fine char" referenced in technical literature.
Biochar is highly stable and has a variety of uses, from a soil amendment that improves water retention to a method for long-term carbon sequestration. It can also be used as a solid fuel.
3. Syngas (The Gas)
The non-condensable gases produced during pyrolysis are collectively known as syngas (synthesis gas).
This gas is a mixture of hydrogen, carbon monoxide, carbon dioxide, and methane. It is combustible and is often recycled to provide the heat needed to power the pyrolysis process itself, making the system more energy-efficient.
Understanding the Trade-offs: Is Bio-oil Truly "More Useful"?
The value of bio-oil is relative. While it concentrates the energy from bulky biomass into a transportable liquid, its direct applications are limited without further refinement.
The Challenge of Raw Bio-oil
Raw bio-oil's high oxygen content, acidity, and instability make it corrosive to standard engines and pipelines. It cannot be blended directly with petroleum fuels and tends to thicken or polymerize over time.
Its most immediate use is as a substitute for heavy fuel oil in stationary applications like industrial boilers or furnaces, where equipment can be adapted to handle it.
The Necessity of Upgrading
To produce "more useful" liquids like transportation fuels (gasoline, diesel), bio-oil must undergo a secondary process called upgrading.
Upgrading typically involves catalytic reactions, such as hydrotreating, to remove oxygen, reduce acidity, and improve its stability. This step adds significant cost and complexity to the overall fuel production chain.
Process Conditions Dictate the Outcome
The yield of liquid, solid, and gas is not fixed. It can be heavily influenced by the pyrolysis conditions:
- Fast Pyrolysis: Rapid heating and short residence times maximize the yield of bio-oil (up to 75% by weight).
- Slow Pyrolysis: Slower heating rates favor the production of biochar, making it the primary product.
This tunability allows operators to target the product stream that is most valuable for their specific goals.
Making the Right Choice for Your Goal
Pyrolysis is not a single solution but a versatile platform. Success depends on having a clear objective for the output materials.
- If your primary focus is producing a liquid fuel intermediate: Use fast pyrolysis to maximize bio-oil yield, but plan for the significant capital and operational costs of an upgrading facility.
- If your primary focus is carbon sequestration or soil improvement: Slow pyrolysis is the superior pathway, as it is optimized for producing stable, high-quality biochar.
- If your primary focus is waste-to-energy on a local scale: View pyrolysis as an integrated system where the syngas powers the unit and the bio-oil and biochar are used for local heating or power generation.
Ultimately, pyrolysis is an effective technology for transforming biomass into a more energy-dense liquid, but unlocking its full potential requires a clear strategy for using all of its products.
Summary Table:
| Product | Description | Key Applications |
|---|---|---|
| Bio-oil | Dark, viscous liquid from condensed vapors | Industrial heating, fuel upgrading |
| Biochar | Solid carbon-rich residue | Soil amendment, carbon sequestration |
| Syngas | Non-condensable gas mixture (H2, CO, CH4) | Process heat, power generation |
Ready to optimize your biomass conversion process? KINTEK specializes in lab equipment and consumables for pyrolysis research and development. Whether you're analyzing bio-oil composition, testing biochar properties, or scaling up reactor systems, our precision tools help you achieve accurate, reproducible results. Contact our experts today to discuss how we can support your laboratory's sustainable energy projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















