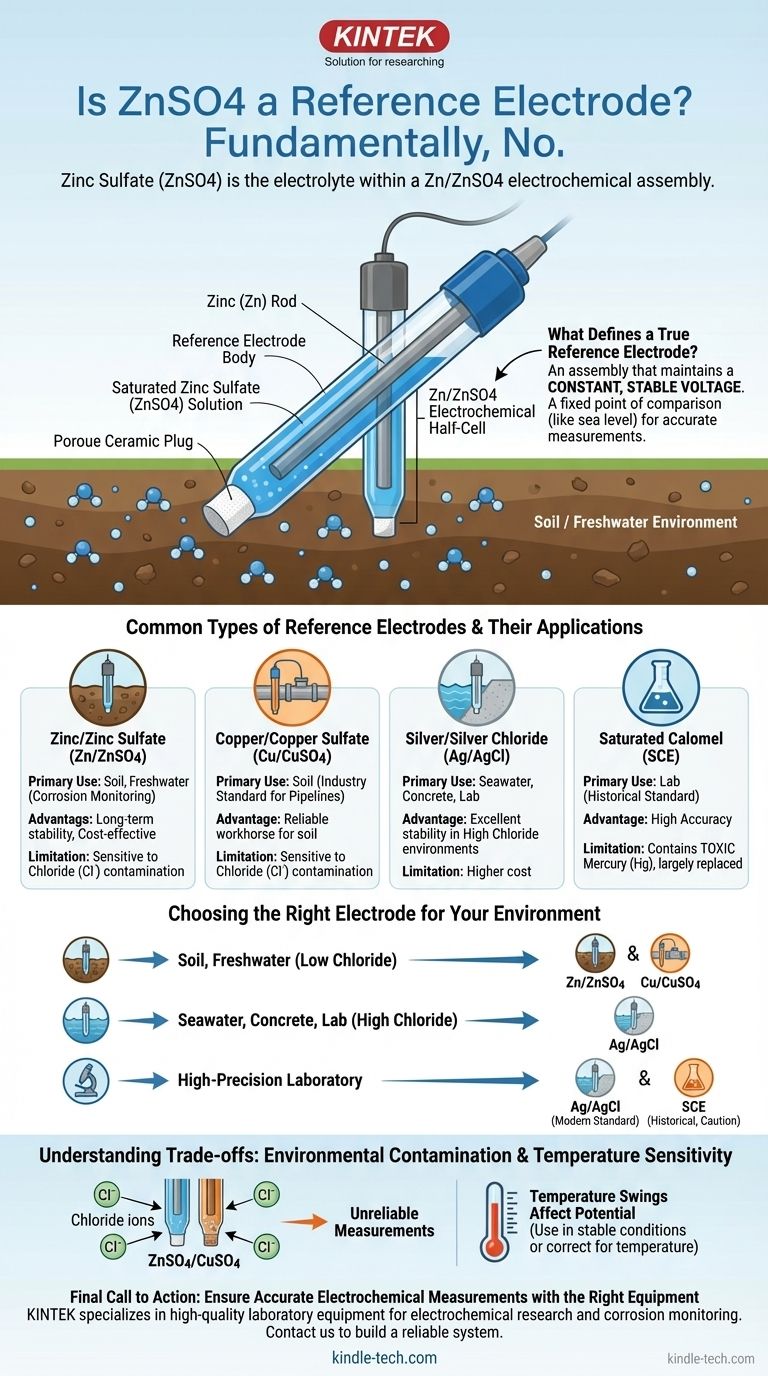
Fundamentally, no. Zinc sulfate (ZnSO4) solution itself is not a reference electrode. It is the electrolyte component within a complete electrochemical assembly known as a Zinc/Zinc Sulfate (Zn/ZnSO4) reference electrode. This specific type of electrode is most often used to measure corrosion potential in soil and freshwater environments.
A reference electrode is not just a chemical solution; it is a complete electrochemical half-cell designed to maintain a constant, stable voltage. The electrode itself is the system of a high-purity zinc rod immersed in a saturated zinc sulfate solution, which then acts as a reliable benchmark for measurements.

What Defines a True Reference Electrode?
To understand the role of ZnSO4, you must first understand the principles of a reference electrode. Its purpose is to provide a stable, known potential (voltage) that acts as a fixed point of comparison, much like "sea level" provides a fixed reference for measuring elevation.
The Principle of Stable Potential
A reference electrode's most critical feature is its stable and reproducible potential. This voltage should not change significantly even when small electrical currents are passed through it, or when the surrounding temperature or chemical composition varies slightly.
This stability allows you to accurately measure the potential of another material (like a steel pipeline) against this known, unchanging benchmark.
The Core Components of a Half-Cell
A reference electrode is a type of electrochemical half-cell. It is an assembly, not a single substance. The Zn/ZnSO4 electrode consists of three key parts:
- The Metallic Element: A high-purity zinc rod.
- The Electrolyte: A saturated solution of zinc sulfate (ZnSO4). This is the role the chemical plays.
- The Porous Junction: A ceramic or wooden plug that allows electrical contact with the environment (e.g., the soil) without letting the internal solution leak out quickly.
Why the Zinc/Zinc Sulfate System is Used
The combination of zinc metal and zinc sulfate solution creates a specific, predictable electrochemical equilibrium. This system is robust, relatively inexpensive, and particularly well-suited for direct burial in soil, which is why it is common in the cathodic protection industry for underground pipelines and tanks.
Common Types of Reference Electrodes
The Zn/ZnSO4 electrode is just one of several common types, each suited for a different environment.
Zinc/Zinc Sulfate (Zn/ZnSO4)
This electrode is the standard for permanent or portable use in soil and fresh water. It is known for its long-term stability in these low-chloride environments.
Copper/Copper Sulfate (Cu/CuSO4)
Very similar in application to the Zn/ZnSO4 electrode, the copper/copper sulfate (CSE) electrode is also a workhorse for measuring potentials in soil. It is arguably the most common reference electrode used for land-based pipelines.
Silver/Silver Chloride (Ag/AgCl)
As noted in references, the Silver-Silver Chloride electrode is extremely stable and widely used. Its key advantage is its excellent performance in environments with high chloride concentrations, such as seawater and concrete.
Saturated Calomel Electrode (SCE)
The Calomel electrode is a highly accurate laboratory standard. However, it contains mercury, which is highly toxic. Due to these environmental and safety concerns, it has been largely replaced by the Ag/AgCl electrode for most applications.
Understanding the Trade-offs and Limitations
No single reference electrode is perfect for all situations. Choosing the wrong one can lead to inaccurate and misleading data.
Environmental Contamination
The primary weakness of Zn/ZnSO4 and Cu/CuSO4 electrodes is chloride contamination. If used in seawater or soil with high salt content (e.g., from de-icing salts), chloride ions will penetrate the porous plug and alter the electrolyte chemistry.
This contamination shifts the electrode's reference potential, making any measurements unreliable. This is precisely why Ag/AgCl is the standard for marine applications.
Temperature Sensitivity
All reference electrodes have some degree of temperature sensitivity. For most field applications, this effect is minor, but it can be significant in environments with wide temperature swings or for high-precision laboratory work.
Maintenance and Lifespan
Portable reference electrodes require periodic checking and refilling of their electrolyte solution to ensure saturation and prevent drying out. Permanent, prepackaged electrodes are designed to be buried and operate for decades without maintenance, but they must be installed correctly to function properly.
Choosing the Right Electrode for Your Application
Your choice must be driven by the chemical environment where the measurement will take place.
- If your primary focus is corrosion monitoring in soil or fresh water: The Zinc/Zinc Sulfate or Copper/Copper Sulfate electrode is the industry-standard choice.
- If your primary focus is working in seawater, brackish water, or chloride-rich concrete: The Silver/Silver Chloride electrode is the only reliable option for accurate measurements.
- If your primary focus is high-precision laboratory work: A laboratory-grade Silver/Silver Chloride electrode is the modern, safe, and stable standard.
Selecting the correct reference electrode is the foundation for all accurate and meaningful electrochemical measurements.
Summary Table:
| Electrode Type | Primary Use Case | Key Advantage | Key Limitation |
|---|---|---|---|
| Zinc/Zinc Sulfate (Zn/ZnSO4) | Soil, Freshwater | Long-term stability, cost-effective | Sensitive to chloride contamination |
| Copper/Copper Sulfate (Cu/CuSO4) | Soil | Industry standard for pipelines | Sensitive to chloride contamination |
| Silver/Silver Chloride (Ag/AgCl) | Seawater, Concrete, Lab | Excellent stability in chloride-rich environments | Higher cost than Zn/ZnSO4 or Cu/CuSO4 |
| Saturated Calomel (SCE) | Lab (Historical) | High accuracy | Contains toxic mercury (largely replaced) |
Ensure Accurate Electrochemical Measurements with the Right Equipment
Selecting the correct reference electrode is critical for reliable data in corrosion monitoring and laboratory analysis. The choice depends entirely on your specific environment—be it soil, freshwater, or seawater.
KINTEK specializes in high-quality laboratory equipment and consumables. We understand the precise needs of electrochemical research and corrosion monitoring. Our expertise can help you select the right reference electrodes and supporting lab equipment to ensure your measurements are accurate and meaningful.
Let us help you build a reliable measurement system. Contact our experts today to discuss your application requirements and find the perfect solution for your laboratory's challenges.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
People Also Ask
- How is a three-electrode electrochemical electrolytic cell utilized to evaluate Zr-Nb alloy corrosion resistance?
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments
- What is the difference between electrolytic corrosion cell and electrochemical corrosion cell? Understand the Driving Force Behind Corrosion
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- What is the volume range of the coating evaluation electrolytic cell? A Guide to Choosing the Right Size



















