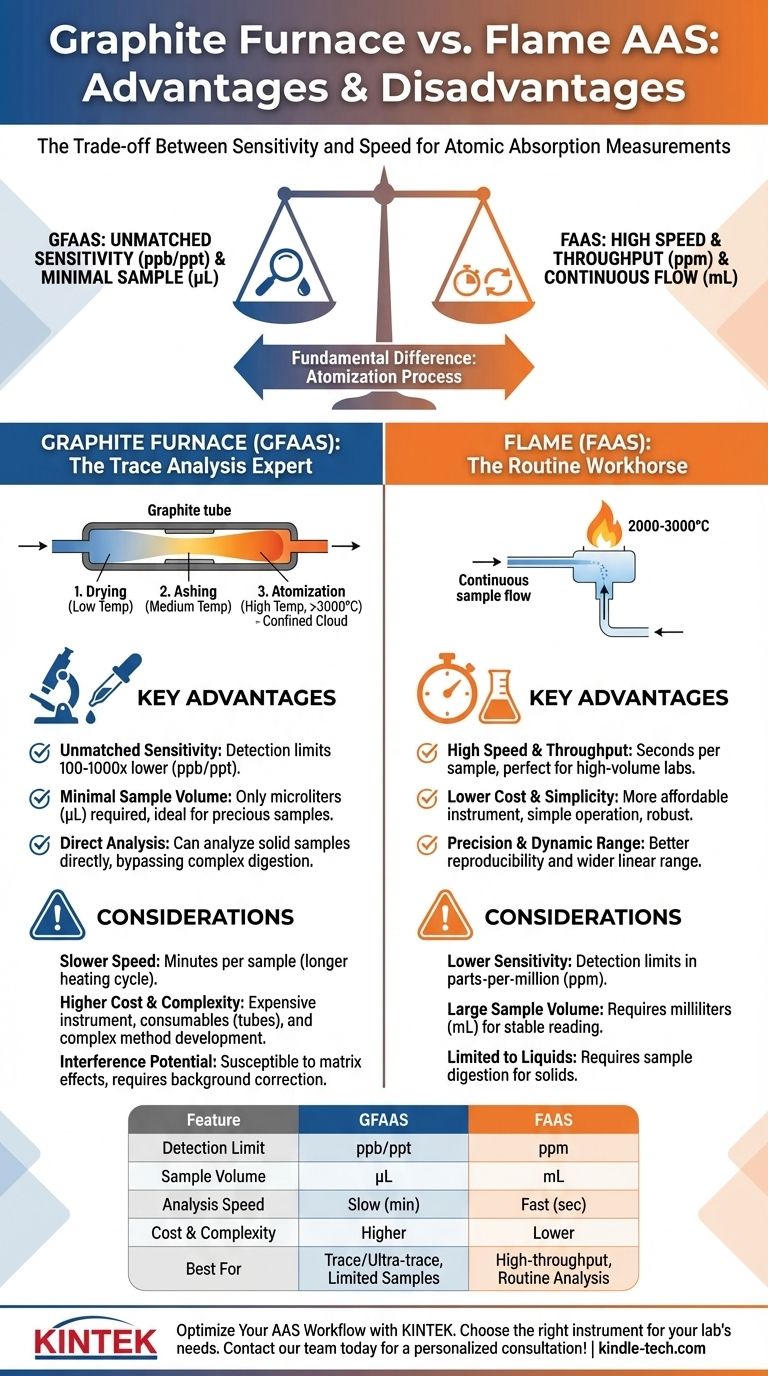
In analytical chemistry, the primary advantage of a graphite furnace over a flame for atomic absorption is its vastly superior sensitivity, allowing for the detection of elements at much lower concentrations. Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) achieves this by confining the sample atoms in the light path for a longer duration, but this benefit comes at the cost of slower analysis times, increased complexity, and higher operational expense compared to Flame Atomic Absorption Spectroscopy (FAAS).
The choice between a graphite furnace and a flame is a fundamental trade-off between sensitivity and speed. GFAAS is the expert tool for trace and ultra-trace analysis where every atom counts, while FAAS is the workhorse for routine, higher-concentration measurements where throughput is key.
The Fundamental Difference: How Atoms Are Made
At the heart of both techniques is the process of atomization—converting a sample into a cloud of free, ground-state atoms that can absorb light. The method of atomization dictates the instrument's performance.
Flame AAS (FAAS): A Continuous Flow
In FAAS, the liquid sample is continuously aspirated through a nebulizer, creating a fine aerosol that is mixed with fuel and oxidant gases. This mixture is then carried into a flame (typically 2000-3000°C).
The instrument measures a steady-state signal as the sample flows, but individual atoms only spend a fraction of a second in the spectrometer's light path before being swept away.
Graphite Furnace AAS (GFAAS): A Discrete Confinement
In GFAAS, a very small, discrete volume of the sample (typically 5-50 µL) is pipetted directly into a graphite tube. The tube is then heated in a pre-programmed sequence:
- Drying: Low temperature to evaporate the solvent.
- Ashing (Pyrolysis): Medium temperature to burn off organic matrix components.
- Atomization: High temperature (up to 3000°C) to vaporize the analyte into a dense cloud of atoms.
This process confines the atom cloud inside the tube and within the light path for several seconds, dramatically increasing the absorption signal.
Key Advantages of Graphite Furnace (GFAAS)
The unique atomization process in GFAAS provides distinct benefits that are essential for specific analytical challenges.
Unmatched Sensitivity
This is the single most important advantage of GFAAS. By holding the atomized sample in the light path longer, GFAAS can achieve detection limits 100 to 1,000 times lower than FAAS.
This allows for measurement at parts-per-billion (ppb) or even parts-per-trillion (ppt) levels, compared to the parts-per-million (ppm) range typical for FAAS.
Minimal Sample Volume
FAAS requires a continuous flow of sample, often consuming several milliliters (mL) for a stable reading. GFAAS requires only a tiny, discrete aliquot, usually measured in microliters (µL).
This is critical when analyzing precious or limited samples, such as clinical blood samples, rare biological tissues, or expensive materials.
Direct Analysis Capability
While most GFAAS analysis is done with liquids, some specialized systems allow for the direct analysis of solid samples. A small mass of the solid can be weighed and placed directly into the furnace, bypassing the need for complex and time-consuming acid digestion required for FAAS.
Understanding the Trade-offs: The Case for Flame AAS
The high sensitivity of GFAAS is not without significant drawbacks. These trade-offs are precisely why FAAS remains a widely used and valuable technique.
Speed and Throughput
An FAAS measurement is fast, taking only a few seconds per sample to get a stable reading. In contrast, a single GFAAS analysis takes several minutes to complete its heating cycle.
For a quality control lab that needs to analyze hundreds of samples per day, the high throughput of FAAS is a decisive advantage.
Cost and Complexity
GFAAS instruments are generally more expensive to purchase than FAAS systems. Furthermore, the graphite tubes are consumables with a finite lifetime of a few hundred firings, representing a significant ongoing operational cost.
The GFAAS method itself is also more complex, requiring careful development of the temperature program for each sample type to manage matrix effects.
Potential for Interference
Because GFAAS atomizes the entire sample aliquot, including the matrix, it is more susceptible to background absorption and chemical interferences. This often necessitates more sophisticated (and expensive) background correction systems, like Zeeman effect correction, to ensure accurate results.
Precision and Dynamic Range
The continuous, steady-state signal of FAAS generally results in better measurement precision (reproducibility) than the transient signal from a GFAAS. Additionally, FAAS typically has a wider linear dynamic range, making it better suited for samples with widely varying concentrations.
Making the Right Choice for Your Goal
The decision to use GFAAS or FAAS is driven entirely by your analytical objective and practical constraints.
- If your primary focus is trace or ultra-trace analysis (ppb/ppt levels): GFAAS is the only viable choice for achieving the required detection limits.
- If your primary focus is high sample throughput and speed: FAAS is vastly superior and the clear choice for production or quality control environments.
- If your primary focus is conserving a limited or precious sample: GFAAS is the necessary technique due to its minimal volume requirement.
- If your primary focus is lower cost, simplicity, and robustness: FAAS is the more practical, economical, and forgiving technique for routine analysis.
Ultimately, your required detection limit is the most critical factor, dictating which technology is a necessity versus a practical choice.

Summary Table:
| Feature | Graphite Furnace (GFAAS) | Flame (FAAS) |
|---|---|---|
| Detection Limit | Parts-per-billion (ppb) to parts-per-trillion (ppt) | Parts-per-million (ppm) |
| Sample Volume | Microliters (µL) | Milliliters (mL) |
| Analysis Speed | Slow (minutes per sample) | Fast (seconds per sample) |
| Cost & Complexity | Higher (instrument & consumables) | Lower & simpler |
| Best For | Trace/ultra-trace analysis, limited samples | High-throughput, routine analysis |
Need to optimize your atomic absorption spectroscopy workflow? The choice between a graphite furnace and a flame source is critical for achieving accurate results efficiently. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the right AAS instrument—whether you require the ultra-low detection limits of GFAAS or the high-speed throughput of FAAS—to enhance your lab's analytical capabilities and productivity. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Large Vertical Graphite Vacuum Graphitization Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- Why is the graphite furnace technique more sensitive than flame based vaporization methods for atomic absorption? Unlock Superior Trace Analysis
- What are the interferences of graphite furnace? Overcome Matrix & Spectral Issues for Accurate GFAAS
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes
- What does graphite furnace measure? A Key Tool for Trace Analysis & High-Temp Processing
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- How does an induction graphitization furnace facilitate the transformation of unburned carbon into synthetic graphite?
- Why is a graphite furnace more sensitive than a flame? Unlocking Superior Trace Analysis
- What is the difference between graphite furnace and flame AAS? Choose the Right Technique for Your Lab



















