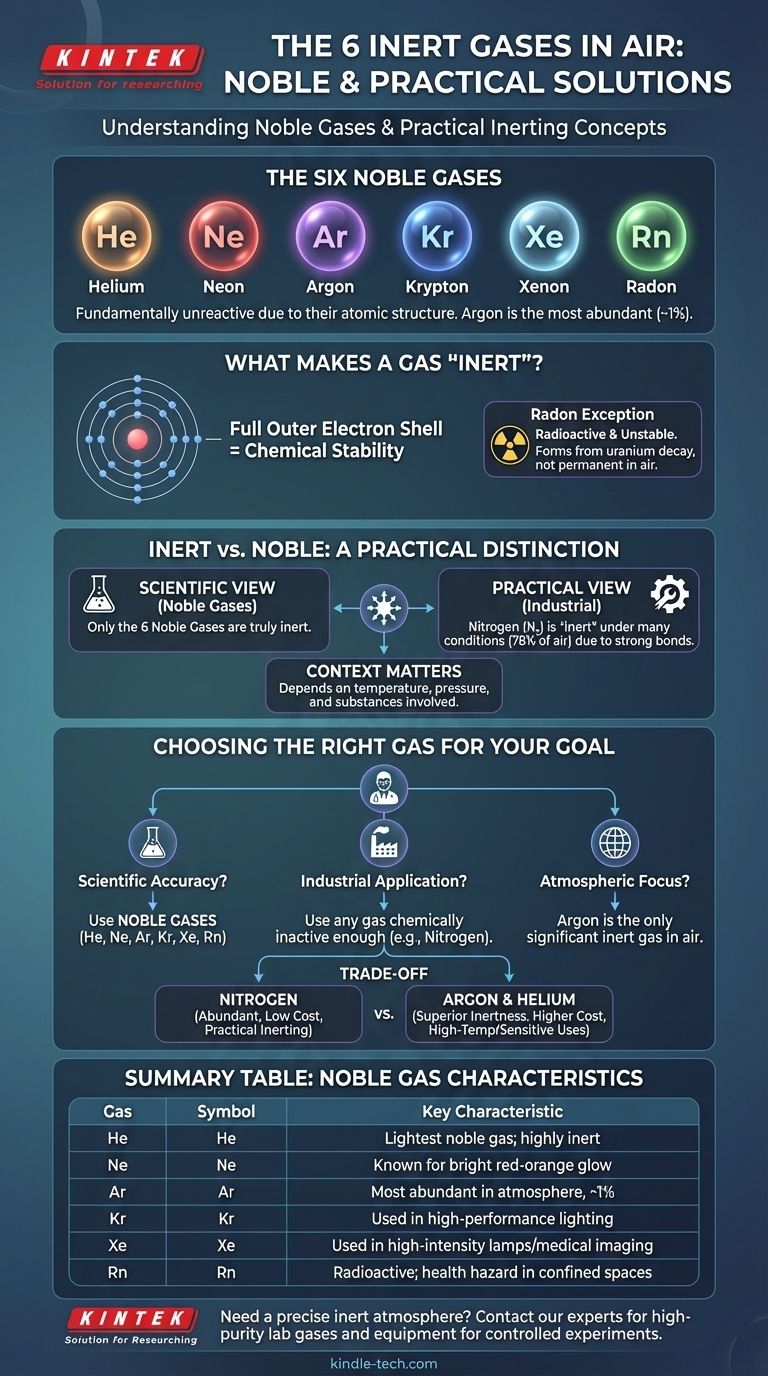
The six noble gases, often referred to as inert gases, are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn). While all are found in the atmosphere, their abundance varies dramatically, with Argon being a significant component and others existing only in trace amounts.
The term "inert gas" has two meanings. Scientifically, it refers to the six noble gases, which are fundamentally unreactive due to their atomic structure. In practice, however, it describes any gas that is non-reactive under specific conditions, which often includes abundant gases like nitrogen.

What Truly Makes a Gas "Inert"?
The concept of "inertness" is rooted in chemical stability. A gas that does not readily participate in chemical reactions is considered inert, but the reason for that stability is the crucial distinction.
The Noble Gases: A Full Electron Shell
The six gases listed—Helium, Neon, Argon, Krypton, Xenon, and Radon—belong to a special group on the periodic table. Their defining characteristic is a full outer shell of electrons.
This stable electron configuration means they have no tendency to gain, lose, or share electrons. This fundamental property makes them inherently and almost universally non-reactive.
Argon: The Most Common Inert Gas in Air
While nitrogen and oxygen dominate our atmosphere, argon is the third most abundant gas, making up nearly 1% of the air we breathe. It is by far the most common of the noble gases in our environment.
Radon: The Unstable Exception
Radon is a noble gas and is chemically inert. However, it is radioactive and forms from the natural decay of uranium in soil and rock. It is not a stable, permanent component of the atmosphere in the same way as the others.
"Inert" vs. "Noble": A Practical Distinction
While a chemist thinks of the six noble gases when they hear "inert," an engineer or manufacturer may have a different perspective. In industrial applications, the goal is simply to prevent unwanted chemical reactions.
A Question of Context
In practice, a gas is considered inert if it doesn't react with the specific materials being used. The degree of inertness needed depends on factors like temperature, pressure, and the substances involved.
Nitrogen: The Practical Workhorse
Nitrogen gas (N₂) makes up 78% of our atmosphere. While it is not a noble gas, the two nitrogen atoms are held together by an incredibly strong triple bond.
Breaking this bond requires a great deal of energy, making nitrogen effectively inert in many common applications, from food packaging to electronics manufacturing. It is only at very high temperatures or pressures that nitrogen becomes reactive with certain materials.
Understanding the Trade-offs
Choosing a gas for creating an inert atmosphere involves balancing performance against practical constraints. The most chemically inert option is not always the best choice.
Reactivity vs. Cost
Nitrogen is abundant and inexpensive, making it the default choice for most industrial inerting applications.
Argon and helium are significantly more inert than nitrogen and are used in high-temperature processes like welding or when working with highly reactive metals. This superior performance comes at a much higher cost.
The Limits of "Inertness"
It is important to recognize that inertness is not absolute. Under extreme laboratory conditions of high pressure and temperature, scientists have successfully forced noble gases like xenon and krypton to form chemical compounds. For all practical purposes, however, they remain unreactive.
Making the Right Choice for Your Goal
Your definition of "inert gas" depends entirely on your context and what you need to achieve.
- If your primary focus is scientific accuracy: The six noble gases (He, Ne, Ar, Kr, Xe, Rn) are the only true inert gases due to their fundamental atomic structure.
- If your primary focus is industrial application: An "inert gas" is any gas, including nitrogen, that is chemically inactive enough for your specific process and budget.
- If your primary focus is atmospheric composition: Argon is the only inert gas present in the air in a significant quantity (almost 1%), with the others existing in trace amounts.
Understanding the difference between fundamental properties and practical application is key to mastering the concept.
Summary Table:
| Gas | Symbol | Key Characteristic |
|---|---|---|
| Helium | He | Lightest noble gas; highly inert |
| Neon | Ne | Known for bright red-orange glow in signs |
| Argon | Ar | Most abundant noble gas in the atmosphere (~1%) |
| Krypton | Kr | Used in high-performance lighting |
| Xenon | Xe | Used in high-intensity lamps and medical imaging |
| Radon | Rn | Radioactive; a health hazard in confined spaces |
Need to create a precise inert atmosphere for your lab? The right choice of gas is critical for the success and safety of your processes, whether you're conducting high-temperature heat treatment, welding, or sensitive material synthesis. KINTEK specializes in providing high-purity lab gases and equipment to ensure your experiments and production runs are perfectly controlled. Contact our experts today to discuss the best inert gas solution for your specific application and ensure optimal results.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Electron Beam Evaporation Coating Conductive Boron Nitride Crucible BN Crucible
- Engineering Advanced Fine Ceramics Aluminium Oxide Al2O3 Ceramic Washer for Wear-Resistant Applications
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
People Also Ask
- At what temperature does graphite thermal decompose? The Critical Role of Atmosphere
- Is a graphite melting point high or low? Discover Its Extreme Thermal Resilience
- How does graphite react to heat? Unlocking Its Unique High-Temperature Strengths
- Can graphite withstand high-temperature? Maximizing Performance in Controlled Atmospheres
- Is graphite good in high temperature? Unlocking Its Extreme Heat Potential











