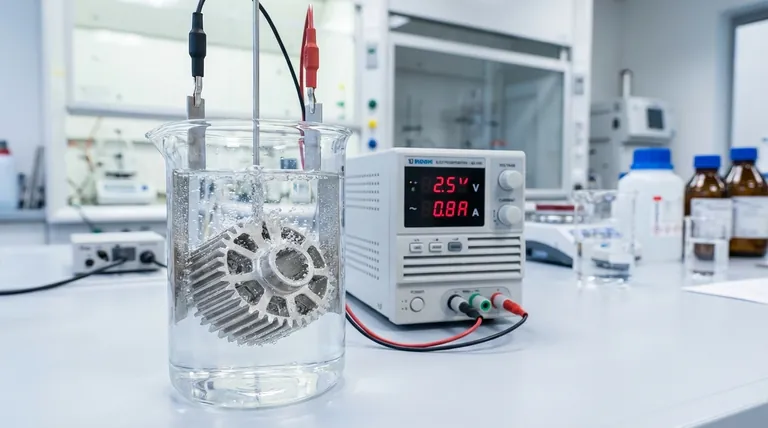
In material science and manufacturing, electrodeposition offers a unique combination of precision, cost-effectiveness, and scalability. This electrochemical process allows for the creation of thin, uniform films on a conductive surface by passing an electric current through an electrolyte solution, enabling the controlled deposition of materials like metals, alloys, and composites.
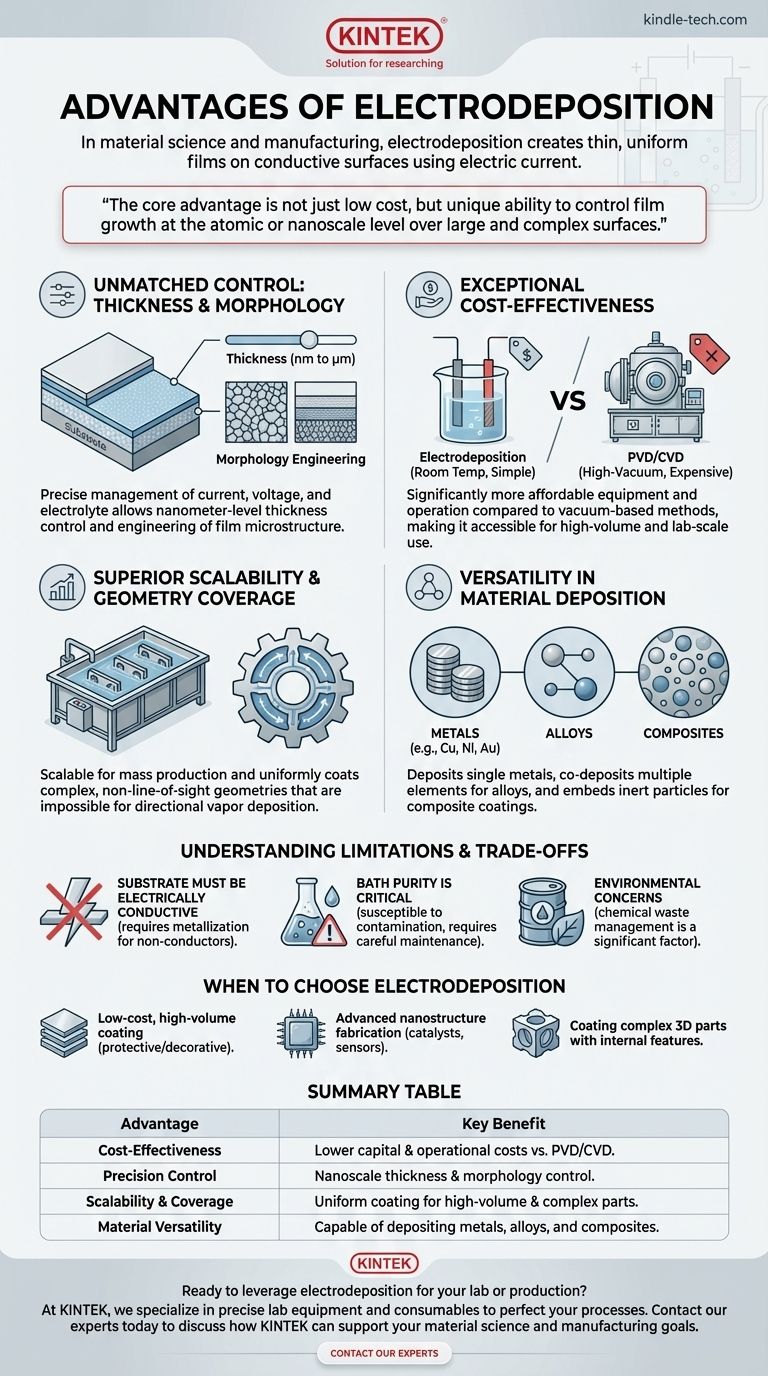
The core advantage of electrodeposition is not just its low cost, but its unique ability to control film growth at the atomic or nanoscale level over large and complex surfaces—a capability often reserved for far more expensive vacuum-based technologies.
The Core Advantages of Electrodeposition
Electrodeposition, often called electroplating in industrial contexts, derives its power from the precise electrochemical control it offers. This control translates into several key benefits for engineers and researchers.
Unmatched Control Over Thickness and Morphology
By carefully managing parameters like current density, voltage, and electrolyte composition, you gain direct control over the deposition rate. This allows for the creation of films with thicknesses ranging from a few nanometers to many micrometers with exceptional uniformity.
This process isn't just about thickness. It enables the engineering of the film's microstructure, or morphology. You can create nanostructured films, control grain size, and even deposit multi-layered structures (nanolaminates) by changing the deposition conditions in real-time.
Exceptional Cost-Effectiveness
Compared to alternative thin-film deposition methods like Physical Vapor Deposition (PVD) or Chemical Vapor Deposition (CVD), electrodeposition is significantly more affordable. The required equipment is relatively simple, operates at or near room temperature, and does not require expensive high-vacuum chambers.
This lower capital and operational cost makes it an accessible technique for both high-volume industrial production and exploratory lab-scale research.
Superior Scalability and Geometry Coverage
Electrodeposition is an inherently scalable process. Once a plating bath chemistry is optimized, it can be applied to coat very large surface areas simultaneously, making it ideal for mass production.
Furthermore, because deposition occurs from a liquid electrolyte, the process can uniformly coat parts with complex, non-line-of-sight geometries. It excels at covering intricate shapes, recessed areas, and internal surfaces that are impossible to reach with directional vapor deposition techniques.
Versatility in Material Deposition
While commonly associated with depositing single metals like copper, nickel, or gold, the technique is highly versatile. It can be adapted to co-deposit multiple elements to form alloys with specific mechanical or chemical properties.
By suspending inert particles (like ceramics) in the electrolyte, you can also form composite coatings, embedding the particles into the growing metal film to enhance properties like wear resistance or hardness.
Understanding the Limitations and Trade-offs
No technique is universally superior. To leverage electrodeposition effectively, it is critical to understand its inherent constraints.
Material and Substrate Constraints
The most significant limitation is that the substrate must be electrically conductive. While techniques exist to metallize non-conductive surfaces (like plastics) prior to plating, it adds an extra, complex step to the process.
Additionally, only materials that can be successfully reduced from an electrolyte solution can be deposited. This excludes many materials and can make depositing highly reactive metals like aluminum or titanium from aqueous solutions very challenging or impossible.
Purity and Bath Maintenance
The electrolyte bath is an open system susceptible to contamination from airborne dust, anode dissolution byproducts, and chemical breakdown. Maintaining the purity and chemical balance of the bath is critical for achieving consistent, high-quality deposits.
Failure to properly manage the bath chemistry can lead to impurities in the final film, which can degrade its mechanical, electrical, or optical properties.
Environmental and Safety Concerns
Many traditional and high-performance plating baths use acidic, alkaline, or toxic chemicals. The management and disposal of this chemical waste are significant environmental and cost considerations that must be factored into the overall process.
When to Choose Electrodeposition
Your choice of deposition technology should be driven by your end goal. Electrodeposition is the optimal choice in several distinct scenarios.
- If your primary focus is low-cost, high-volume coating: Electrodeposition is unmatched for applying protective or decorative metal layers on products like fasteners, automotive trim, or plumbing fixtures.
- If your primary focus is advanced nanostructure fabrication: The precise control over thickness and morphology makes it a powerful tool for creating materials for catalysts, sensors, or battery electrodes.
- If your primary focus is coating complex 3D parts: Use electrodeposition for components with internal channels, threads, or intricate surface features where uniform coverage is essential.
Ultimately, electrodeposition provides a powerful and accessible bridge between bulk manufacturing and nanoscale engineering.
Summary Table:
| Advantage | Key Benefit |
|---|---|
| Cost-Effectiveness | Lower capital & operational costs vs. PVD/CVD methods. |
| Precision Control | Nanoscale thickness & morphology control for advanced materials. |
| Scalability & Coverage | Uniform coating for high-volume production and complex 3D parts. |
| Material Versatility | Capable of depositing metals, alloys, and composite coatings. |
Ready to leverage electrodeposition for your lab or production line?
At KINTEK, we specialize in providing the precise lab equipment and consumables needed to perfect your electrodeposition processes. Whether you are developing advanced nanostructured materials or scaling up a coating application for complex parts, our expertise ensures you achieve consistent, high-quality results.
We understand the critical balance between cost, precision, and scalability. Let us help you optimize your deposition parameters and maintain bath chemistry for superior performance.
Contact our experts today to discuss how KINTEK can support your material science and manufacturing goals.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating
- How is something diamond coated? A Guide to CVD Growth vs. Plating Methods
- What is the role of the HF-CVD system in preparing BDD electrodes? Scalable Solutions for Boron-Doped Diamond Production
- What is microwave plasma CVD? A Guide to High-Purity Diamond and Material Synthesis
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth



















