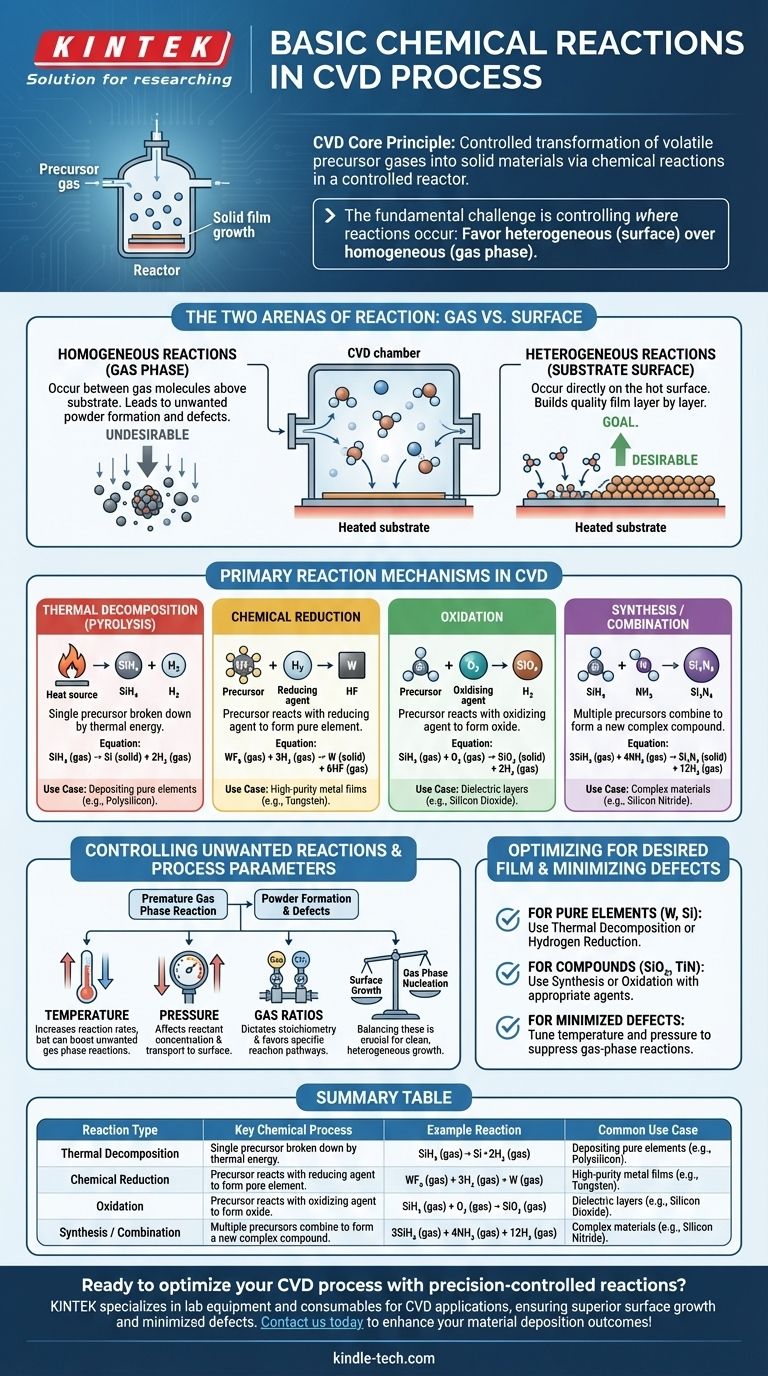
At its core, Chemical Vapor Deposition (CVD) is a process of controlled chemical reactions. These reactions are designed to take stable, volatile precursor gases and transform them into a solid, non-volatile material directly onto a substrate. The most common mechanisms driving this transformation are thermal decomposition (pyrolysis), chemical reduction, oxidation, and synthesis reactions, all occurring within a highly controlled reactor environment.
The fundamental challenge in any CVD process is not just initiating chemical reactions, but precisely controlling where they occur. The goal is to favor heterogeneous reactions on the substrate surface to build a quality film, while minimizing homogeneous reactions in the gas phase that create unwanted particles.

The Two Arenas of Reaction: Gas vs. Surface
Every chemical reaction in a CVD chamber happens in one of two places. The balance between them determines the quality and structure of the final material.
Homogeneous Reactions (In the Gas Phase)
These reactions occur between gas molecules themselves, suspended in the space above the substrate.
While some gas-phase chemistry is necessary to create reactive intermediate species, excessive homogeneous reactions are often undesirable. They can lead to the formation of solid particles or "powders" that then rain down on the substrate, causing defects and compromising film quality.
Heterogeneous Reactions (On the Substrate Surface)
These are the reactions that build the film. They happen directly on, or in a very thin layer adjacent to, the heated substrate surface.
The goal of a well-designed CVD process is to maximize the rate of heterogeneous reactions. Precursor gases adsorb onto the hot surface, decompose or react with other adsorbed species, and form the stable solid film one atomic layer at a time.
Primary Reaction Mechanisms in CVD
While happening in one of the two "arenas" above, the reactions themselves can be categorized into several key types based on the chemical transformation involved.
Thermal Decomposition (Pyrolysis)
This is the simplest and most common CVD reaction type. A single precursor gas is broken down into its constituent parts by thermal energy alone.
The heat from the substrate provides the energy needed to break the chemical bonds of the precursor molecule, leaving the desired solid element to deposit on the surface. A classic example is the deposition of polysilicon from silane gas.
SiH₄ (gas) → Si (solid) + 2H₂ (gas)
Chemical Reduction
In this process, a precursor gas (often a metal halide) reacts with a reducing agent, typically hydrogen (H₂), to form a pure elemental film.
This is a common method for depositing high-purity metal films like tungsten. The hydrogen strips the halogen atoms from the metal precursor, allowing the pure metal to deposit.
WF₆ (gas) + 3H₂ (gas) → W (solid) + 6HF (gas)
Oxidation
This reaction involves a precursor gas reacting with an oxidizing agent, such as oxygen (O₂), nitrous oxide (N₂O), or steam (H₂O), to form a solid oxide film.
This is the foundational process for creating insulating dielectric layers like silicon dioxide (SiO₂), a critical component in nearly all modern microelectronics.
SiH₄ (gas) + O₂ (gas) → SiO₂ (solid) + 2H₂ (gas)
Synthesis or Combination
Here, two or more precursor gases are introduced to combine and form a new compound material on the substrate. This allows for the creation of complex materials that cannot be formed by simple decomposition.
For example, silicon nitride (Si₃N₄), a hard and chemically resistant material, is formed by reacting a silicon source with a nitrogen source, like ammonia.
3SiH₄ (gas) + 4NH₃ (gas) → Si₃N₄ (solid) + 12H₂ (gas)
Understanding the Trade-offs: Controlling Unwanted Reactions
The success of a CVD process depends entirely on controlling the reaction environment to favor the desired chemical pathways.
The Problem of Powder Formation
The primary pitfall in CVD is unintended gas-phase nucleation. If the reactor temperature is too high or the pressure is too great, precursor gases can react prematurely in the gas phase (homogeneous reaction) before they reach the substrate. This creates particles that can cause defects or form a low-density, powdery film instead of a high-quality, dense one.
The Role of Process Parameters
Engineers use several key parameters as levers to control the reaction kinetics and location:
- Temperature: Increases reaction rates but can also increase unwanted gas-phase reactions.
- Pressure: Affects the concentration of reactants and how quickly they travel to the surface.
- Gas Ratios: Dictates the stoichiometry and controls which reaction pathway is favored.
Balancing these factors is crucial for promoting clean, heterogeneous growth on the substrate surface.
Optimizing Reactions for Your Desired Film
The specific chemical reaction pathway you employ is determined entirely by the material you intend to create.
- If your primary focus is depositing a pure element (e.g., Tungsten, Silicon): You will likely rely on thermal decomposition or a hydrogen reduction reaction using a single precursor and possibly a reducing agent.
- If your primary focus is creating a compound oxide or nitride (e.g., SiO₂, TiN): You will use a synthesis or oxidation reaction, introducing an oxidizing or nitriding agent alongside your main precursor.
- If your primary focus is minimizing defects and achieving a high-quality film: Your main task is tuning temperature and pressure to suppress homogeneous gas-phase reactions and promote clean, heterogeneous growth on the substrate.
Ultimately, mastering CVD is mastering the art of directing chemistry to occur at a specific time and in a specific place.
Summary Table:
| Reaction Type | Key Chemical Process | Example Reaction | Common Use Case |
|---|---|---|---|
| Thermal Decomposition (Pyrolysis) | Single precursor breaks down via heat | SiH₄ (gas) → Si (solid) + 2H₂ (gas) | Depositing pure elements like polysilicon |
| Chemical Reduction | Precursor reacts with a reducing agent (e.g., H₂) | WF₆ (gas) + 3H₂ (gas) → W (solid) + 6HF (gas) | High-purity metal films (e.g., tungsten) |
| Oxidation | Precursor reacts with an oxidizing agent (e.g., O₂) | SiH₄ (gas) + O₂ (gas) → SiO₂ (solid) + 2H₂ (gas) | Dielectric layers like silicon dioxide |
| Synthesis/Combination | Multiple precursors combine to form a compound | 3SiH₄ (gas) + 4NH₃ (gas) → Si₃N₄ (solid) + 12H₂ (gas) | Complex materials like silicon nitride |
Ready to optimize your CVD process with precision-controlled reactions? KINTEK specializes in lab equipment and consumables for CVD applications, helping you achieve defect-free thin films through tailored reactor solutions. Whether you're depositing metals, oxides, or nitrides, our expertise ensures superior surface growth and minimized gas-phase defects. Contact us today to discuss how our CVD systems can enhance your material deposition outcomes!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained



















