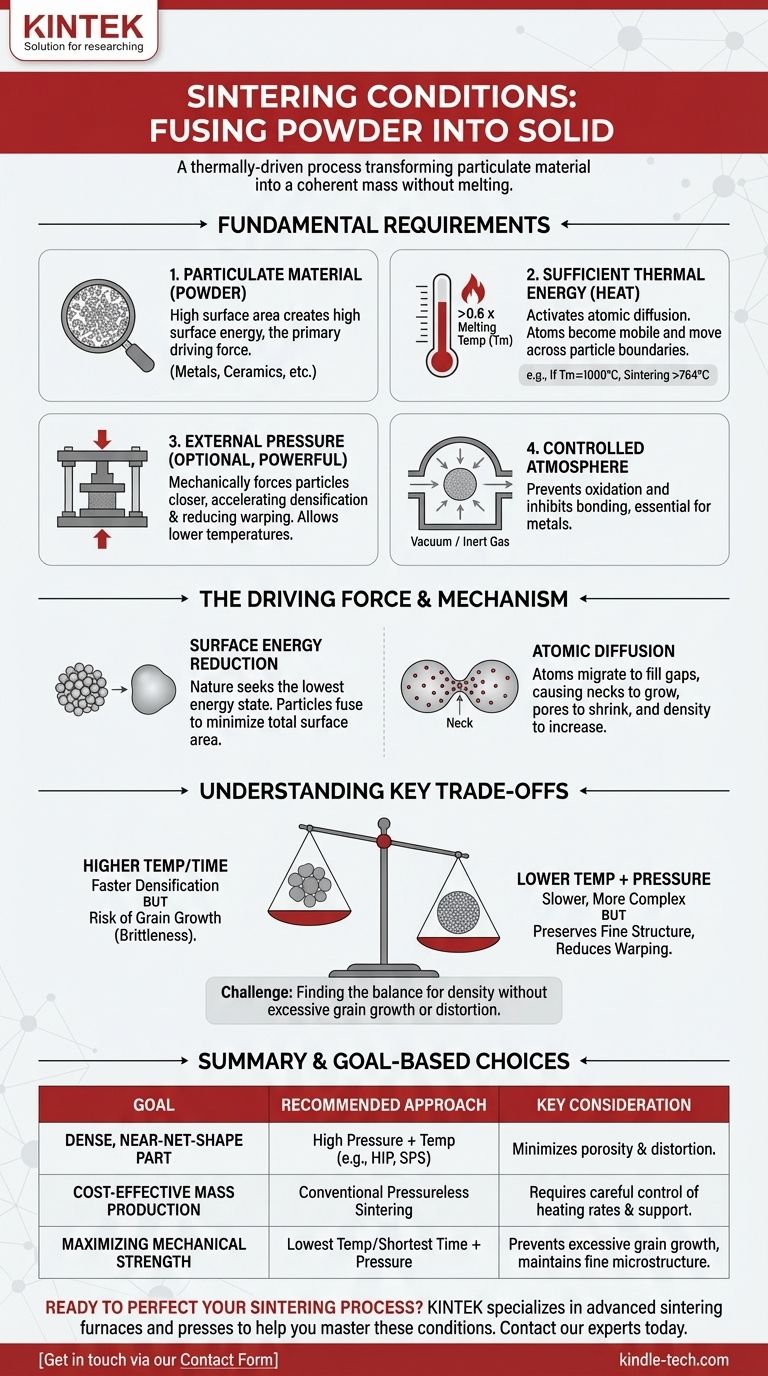
At its core, sintering is a thermally-driven process that requires a specific set of conditions to transform a collection of particles into a solid, coherent mass. The primary conditions are a starting material in a powdered or particulate form and a temperature high enough to enable atomic movement, but low enough to avoid melting the material. External pressure is often applied to assist and accelerate the process.
Sintering should not be confused with melting. The fundamental condition is providing enough thermal energy—not to liquefy the material—but to energize its atoms, allowing them to diffuse across particle boundaries and fuse the powder into a dense, solid object.

The Fundamental Requirements for Sintering
For sintering to occur, three primary conditions must be met: the right material form, sufficient thermal energy, and often, the application of pressure in a controlled environment.
A Particulate Starting Material
Sintering begins with a mass of solid particles, typically a fine powder. This form is essential because it provides a high total surface area.
This high surface area creates a state of high surface energy. The reduction of this energy is the fundamental driving force behind the entire sintering process. The material can be a metal, ceramic, plastic, or a composite.
Sufficient Thermal Energy (Heat)
Heat is the most critical catalyst for sintering. It provides the energy needed to initiate atomic diffusion.
As a rule of thumb, the sintering temperature for most materials is greater than 0.6 times their absolute melting temperature (Tm). For example, if a metal melts at 1000°C (1273 K), its sintering process will typically begin above 764°C (1273 K * 0.6).
This temperature makes the atoms within the crystal lattice mobile enough to move from one particle to another where they touch, gradually fusing them together.
The Role of External Pressure
While not always mandatory, pressure is a powerful tool in sintering. It mechanically forces particles into closer contact.
This close contact reduces the distance atoms need to diffuse, which can significantly speed up the densification process or allow it to occur at a lower temperature. This is the principle behind techniques like Hot Isostatic Pressing (HIP).
A Controlled Atmosphere
In many industrial applications, the atmosphere in which sintering occurs is a critical condition. For metals, a vacuum or an inert gas atmosphere (like argon) is often required to prevent oxidation, which would inhibit proper bonding between particles.
The Driving Force: Why Sintering Actually Happens
Understanding the conditions is useful, but understanding the underlying physical principle is what empowers true process control. Sintering is fundamentally a process of energy minimization.
The Principle of Surface Energy Reduction
A fine powder has an enormous amount of surface area relative to its volume, which corresponds to a high state of surface energy. Nature inherently seeks the lowest possible energy state.
Just as a water droplet naturally forms a sphere to minimize its surface area, a collection of particles under heat will fuse together to reduce its total surface area and, therefore, its total energy.
The Mechanism of Atomic Diffusion
The "magic" of sintering happens at the atomic level. At high temperatures, atoms at the contact points between particles (called "necks") become mobile.
These atoms migrate to fill the gaps and pores between the particles. This causes the necks to grow and the particles to merge, shrinking the overall volume and increasing the density of the component.
Understanding the Trade-offs
The interplay between temperature, pressure, and time presents critical trade-offs that determine the properties of the final product.
Temperature vs. Time
Higher temperatures accelerate diffusion and densification, but they also promote grain growth. If grains within the material grow too large, the final part can become brittle. A key challenge is finding the temperature that achieves density quickly without excessive grain growth.
Warping and Shrinkage
During conventional, pressureless sintering, the part shrinks as pores are eliminated. This shrinkage can be non-uniform, leading to warping or distortion, especially in complex geometries. This is due to factors like gravity and friction with the furnace support.
Pressure as a Solution and a Complication
Applying high pressure can solve many issues. It allows for lower sintering temperatures (preserving a fine grain structure) and ensures more uniform densification, minimizing warping. However, it requires significantly more complex and expensive equipment.
Making the Right Choice for Your Goal
The optimal sintering conditions are dictated entirely by the desired outcome for the final component.
- If your primary focus is creating a dense, near-net-shape part: Use a process that combines high pressure and temperature (like HIP or Spark Plasma Sintering) to minimize porosity and distortion.
- If your primary focus is cost-effective mass production: Conventional pressureless sintering is often the most economical choice, but it requires careful control of heating rates and part support to manage shrinkage.
- If your primary focus is maximizing mechanical strength: Use the lowest possible temperature and shortest time that still achieves the target density, often with applied pressure, to prevent excessive grain growth and maintain a fine microstructure.
Ultimately, mastering sintering is a matter of precisely controlling energy and pressure to guide atomic movement toward your desired material outcome.
Summary Table:
| Condition | Purpose | Key Consideration |
|---|---|---|
| Powdered Material | Provides high surface energy as the driving force | Material type (metal, ceramic, etc.) and particle size |
| Heat (>0.6 x Melting Temp) | Enables atomic diffusion for particle bonding | Balance between densification and grain growth |
| External Pressure | Accelerates process, improves density, reduces warping | Increases equipment complexity and cost |
| Controlled Atmosphere | Prevents oxidation and ensures proper bonding | Required for reactive materials like metals |
Ready to perfect your sintering process and achieve superior material properties?
The precise control of temperature, pressure, and atmosphere is critical for producing dense, strong, and reliable components. KINTEK specializes in advanced lab equipment, including sintering furnaces and presses, to help you master these conditions.
We provide the tools and expertise to optimize your process, whether your goal is cost-effective mass production or maximizing mechanical strength. Contact our experts today to discuss your specific needs and discover how our solutions can enhance your results.
Get in touch via our Contact Form to start the conversation!
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- How does a vacuum environment system contribute to the hot pressing sintering of B4C-CeB6? Unlock Peak Ceramic Density
- What are the advantages of vacuum sintering? Achieve Superior Purity, Strength, and Performance
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- What are the advantages of using a vacuum hot pressing furnace? Achieve 98.9% Density in Al2O3-TiC Laminated Ceramics
- What are the advantages of using a vacuum hot pressing sintering furnace? Superior Density for Nanocrystalline Fe3Al



















