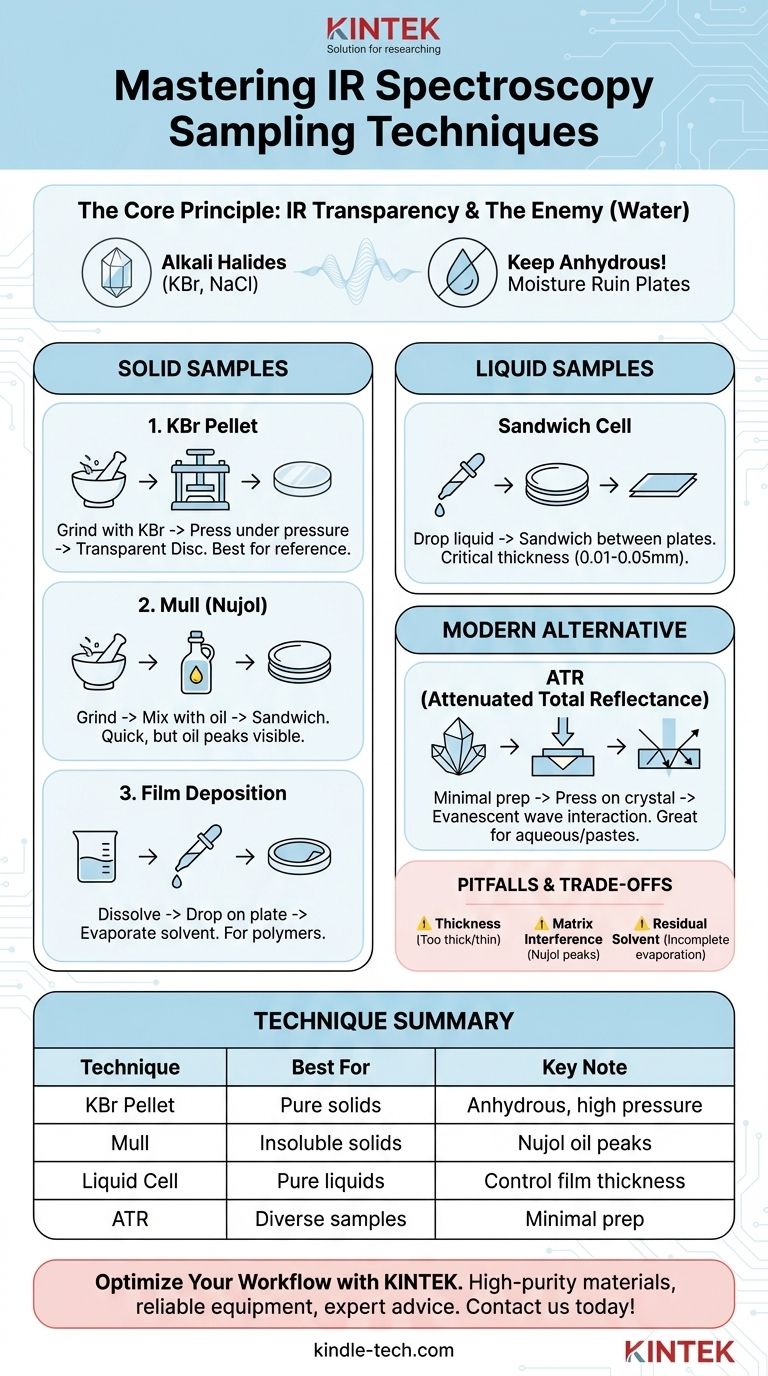
The primary sampling techniques in IR spectroscopy involve preparing the sample in a matrix that is transparent to infrared radiation. For solid samples, this includes the pressed pellet (KBr) technique, the mull technique, and film deposition. For liquid samples, the standard method is to sandwich a thin layer between two salt plates.
The core challenge in IR sample preparation is to get a sufficient amount of your sample into the instrument's beam path without the sample holder or matrix absorbing the IR radiation itself. Your choice of technique is therefore a strategic decision based on your sample's physical state and the need for this "infrared invisibility."

The Fundamental Principle: Infrared Transparency
Why We Use Salt
The material containing your sample must be transparent in the mid-infrared region where molecular vibrations are measured. Common glass or quartz absorbs IR radiation strongly, making it opaque and unusable.
For this reason, sample holders and matrices are typically made from alkali halides. These ionic salts, such as Sodium Chloride (NaCl) and Potassium Bromide (KBr), have no molecular vibrations in the mid-IR range, rendering them effectively invisible to the instrument.
The Enemy of Salt Plates: Water
A critical characteristic of these salts is that they are highly soluble in water. Any moisture in your sample or cleaning solvents will immediately begin to fog, etch, or dissolve the salt plates, ruining them. Therefore, all samples and reagents used in transmission IR spectroscopy must be anhydrous (free of water).
Preparing Solid Samples
When analyzing solids, the goal is to reduce the particle size to prevent light scattering and suspend the sample in an IR-transparent medium.
The Pressed Pellet (KBr) Technique
This method involves thoroughly grinding a small amount of the solid sample with a high-purity, dry powder, most commonly Potassium Bromide (KBr).
The mixture is then placed into a die and compressed under immense pressure. This fuses the KBr into a thin, transparent disc (or pellet) with the sample material evenly dispersed within it. This pellet can be placed directly in the spectrometer's sample holder.
The Mull Technique
In this technique, the solid sample is ground into a fine powder and then mixed with a few drops of a mulling agent, typically a mineral oil like Nujol.
This creates a thick, uniform paste, or "mull." A small amount of this paste is then spread thinly between two flat salt plates (e.g., NaCl plates) to be analyzed.
The Film Deposition Technique
This method is ideal for amorphous solids or polymers that can be dissolved in a volatile solvent.
The solid is dissolved in a suitable non-aqueous solvent. A drop of this solution is placed onto the surface of a single salt plate, and the solvent is gently evaporated. This leaves behind a thin, solid film of the sample on the plate, which is then ready for analysis.
Preparing Liquid Samples
The Sandwich Cell Method
Analyzing liquids is often more straightforward. A drop of the pure liquid is placed on the face of one salt plate.
A second salt plate is then carefully placed on top, spreading the liquid into a very thin film. The path length—the thickness of the liquid layer—is critical and typically ranges from 0.01 to 0.05 mm to ensure the signal is not too strong or too weak.
A Modern Alternative: Attenuated Total Reflectance (ATR)
How ATR Simplifies alysis
ATR is a popular modern technique that avoids many of the challenges of traditional transmission methods. It requires minimal to no sample preparation.
The sample (either solid or liquid) is simply pressed into firm contact with a high-refractive-index crystal, often diamond. The IR beam is directed through the crystal in such a way that it reflects internally, creating an "evanescent wave" that penetrates a few micrometers into the sample at the point of contact. This interaction provides the spectrum.
The Advantages of ATR
Because the IR beam never passes through the bulk sample or a salt plate, ATR can be used for a much wider range of samples. This includes aqueous solutions, thick or opaque solids, and pastes, making it exceptionally versatile and fast.
Understanding the Trade-offs and Pitfalls
Sample Thickness and Concentration
For transmission methods, getting the concentration right is crucial. If the sample film or pellet is too thick or concentrated, it will absorb all the IR light, resulting in a "flat-topped" or useless spectrum. If it is too thin, the signal will be too weak to interpret.
Interference from the Matrix
The mulling agent in the mull technique is itself an organic compound (mineral oil) and will show its own C-H stretching and bending peaks in the spectrum. An analyst must be able to distinguish these known Nujol peaks from the sample's peaks.
Incomplete Solvent Evaporation
When preparing a film from a solution, any residual solvent that has not fully evaporated will also appear in the spectrum, potentially obscuring important sample peaks. It is vital to ensure the film is completely dry.
Selecting the Right Technique for Your Sample
- If your primary focus is a pure, dry solid and you need a high-quality reference spectrum: The KBr pellet technique is the gold standard, as it provides a clear spectrum with no matrix interference.
- If your primary focus is an insoluble or sensitive solid that cannot be ground with KBr: The mull technique is a practical and quick alternative, provided you can account for the mulling agent's peaks.
- If your primary focus is a pure, non-aqueous liquid: A liquid sandwich cell is the simplest and most direct method.
- If your primary focus is rapid analysis of diverse samples (solids, liquids, or aqueous solutions) with minimal prep: ATR is the most efficient and versatile choice in a modern lab.
Mastering these techniques transforms IR spectroscopy from a complex procedure into a powerful and accessible analytical tool.
Summary Table:
| Technique | Best For | Key Consideration |
|---|---|---|
| KBr Pellet | Pure, dry solids; high-quality reference spectra | Requires anhydrous conditions and high pressure |
| Mull (Nujol) | Insoluble or pressure-sensitive solids | Nujol oil peaks appear in the spectrum |
| Liquid Cell | Pure, non-aqueous liquids | Critical to control liquid film thickness (path length) |
| ATR | Rapid analysis of solids, liquids, pastes, and aqueous solutions | Minimal sample preparation required |
Ready to optimize your IR spectroscopy workflow?
The right sampling technique is critical for accurate, high-quality results. KINTEK specializes in providing the lab equipment and consumables you need to master these methods, from KBr pellets and salt plates to durable ATR accessories.
Let our experts help you:
- Select the ideal technique for your specific samples (solids, liquids, or aqueous solutions).
- Source high-purity materials like KBr and NaCl plates to ensure clear, interference-free spectra.
- Improve your lab's efficiency with reliable equipment that simplifies sample preparation.
Contact us today for a consultation and let KINTEK be your partner in achieving precise analytical results.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What is similar to brazing? A Guide to Soldering, Welding, and Thermal Joining
- Does pyrolysis affect the environment? A Dual-Sided Look at Waste Reduction and Pollution Risks
- Why is post-heat treatment required for Li5La3Nb2O12 pellets after SPS? Ensure Material Purity & Stoichiometry
- What is the voltage for arcing? It's Not a Single Number—It's About Electric Field Strength
- What is solid state sintering? A Guide to High-Purity Material Consolidation
- What should be considered when comparing ultra-low freezer models? A Guide to Sample Security, Cost, and Usability
- What are the advantages of using a gas-phase reaction device with reflux condensation for g-C3N4 amination?
- What is the difference between electric arc furnace and plasma arc furnace? Choose the Right Tool for Your Heat Processing Needs



















