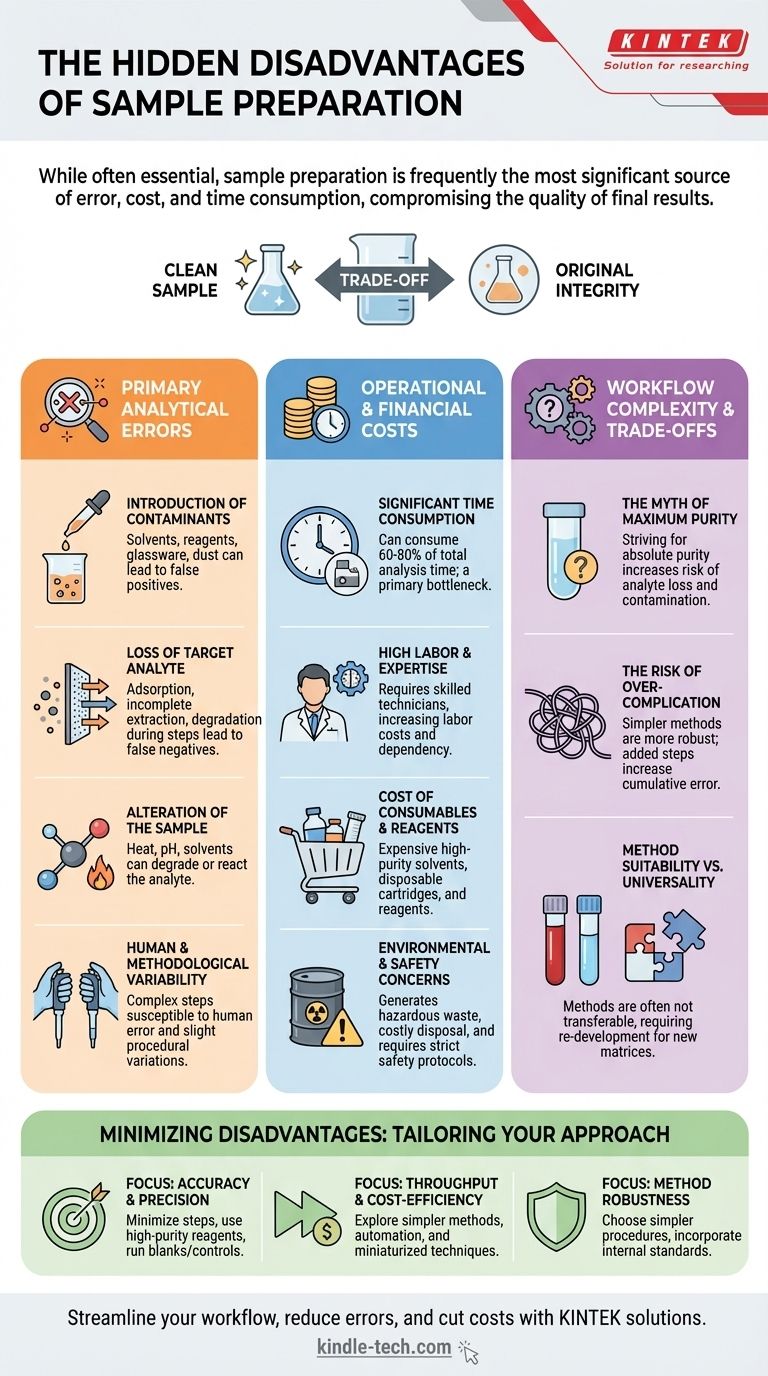
While often essential, sample preparation is frequently the most significant source of error, cost, and time consumption in any analytical workflow. It is a necessary step to isolate analytes and remove interfering substances, but it introduces a host of potential problems that can compromise the quality and reliability of the final result. The disadvantages are not just minor inconveniences; they can fundamentally invalidate an entire analysis.
The core issue with sample preparation is that every step added to a procedure—be it extraction, filtration, or concentration—is another opportunity to introduce contaminants, lose the target analyte, and increase variability. It represents a fundamental trade-off between achieving a "clean" sample and preserving the sample's original integrity.

The Primary Sources of Analytical Error
The most critical disadvantage of sample preparation is its potential to degrade the accuracy and precision of a measurement. Even the most advanced analytical instrument cannot correct for errors introduced before the sample is even loaded.
Introduction of Contaminants
Every material that touches the sample is a potential source of contamination. This includes solvents, reagents, glassware, pipette tips, and even airborne dust in the laboratory. These contaminants can lead to artificially high readings (false positives) or interfere with the detection of the actual target analyte.
Loss of Target Analyte
Conversely, steps designed to remove interferences can also inadvertently remove the substance you are trying to measure. Analyte can be lost through adsorption onto glassware or filter membranes, incomplete extraction from the original matrix, or degradation during heating or evaporation steps. This leads to artificially low results (false negatives).
Alteration of the Sample
The chemical and physical manipulations involved in sample prep can change the analyte itself. Exposure to heat, extreme pH, organic solvents, or even light can cause the target molecule to degrade or react, forming a different substance that will not be detected by the analytical method.
Human and Methodological Variability
Complex, multi-step preparation procedures are highly susceptible to human error. Small variations in how different technicians perform a step—such as mixing time, temperature, or precise volume measurements—can lead to significant differences in the final result, damaging the reproducibility and reliability of the data.
The Hidden Costs of Preparation
Beyond the risks to data quality, sample preparation carries substantial operational and financial burdens that are often underestimated.
Significant Time Consumption
In many analytical labs, sample preparation is the primary bottleneck. It is common for the prep work to consume 60-80% of the total analysis time, while the highly automated instrumental analysis takes only a fraction of that.
High Labor and Expertise Requirements
Properly executing complex preparation protocols requires skilled, well-trained technicians. This represents a significant labor cost and creates a dependency on specific personnel, whose absence can halt laboratory operations.
Cost of Consumables and Reagents
Sample preparation consumes a large quantity of resources. This includes expensive high-purity solvents, disposable items like solid-phase extraction (SPE) cartridges and filters, and specialty reagents. These costs add up quickly, especially in high-throughput environments.
Environmental and Safety Concerns
Many common sample preparation techniques, particularly those involving liquid-liquid or solid-phase extraction, generate significant volumes of hazardous organic solvent waste. The disposal of this waste is costly and carries an environmental burden, while its handling requires strict safety protocols to protect laboratory personnel.
Understanding the Trade-offs: Complexity vs. Quality
The goal is not always to create the "cleanest" possible sample. An effective analyst understands the balance between the benefits of a prep step and the risks it introduces.
The Myth of Maximum Purity
While removing interferences is important, striving for absolute sample purity is often counterproductive. Each additional purification step increases the risk of analyte loss and contamination. A "good enough" preparation that adequately reduces interference without compromising the analyte is often the superior strategy.
The Risk of Over-Complication
Simpler methods are generally more robust. A protocol with ten intricate steps has far more opportunities for cumulative error than a straightforward three-step process. Every added step compounds the potential for variability and failure.
Method Suitability vs. Universality
A highly optimized sample preparation method might work perfectly for one sample type (e.g., blood plasma) but fail completely for another (e.g., wastewater). This lack of transferability means significant time and resources must be spent re-developing and re-validating methods for each new matrix.
Minimizing Disadvantages in Your Workflow
The key is to view sample preparation not as a chore to be rushed, but as an integral part of the measurement itself. Your approach should be tailored to your primary analytical goal.
- If your primary focus is accuracy and precision: Prioritize minimizing the number of steps, using the highest purity reagents, and running procedural blanks and controls alongside every batch to quantify contamination and loss.
- If your primary focus is throughput and cost-efficiency: Explore simpler methods like "dilute-and-shoot," investigate automated sample preparation systems to improve consistency, or adopt miniaturized techniques (e.g., SPME) that use fewer consumables.
- If your primary focus is method robustness: Choose simpler, more forgiving procedures that are less dependent on operator skill and always incorporate an internal standard early in the process to correct for variable analyte recovery.
Ultimately, consciously managing the inherent risks of sample preparation is the defining characteristic of a reliable and efficient analytical process.
Summary Table:
| Disadvantage Category | Key Issues |
|---|---|
| Analytical Error | Contamination, Analyte Loss, Sample Alteration, Human Variability |
| Operational Cost | High Time Consumption, Labor-Intensive, Expensive Consumables |
| Workflow Complexity | Risk of Over-Complication, Lack of Method Universality |
Tired of sample preparation bottlenecks compromising your lab's efficiency and data quality? KINTEK specializes in lab equipment and consumables designed to streamline your workflow, reduce errors, and cut costs. From high-purity reagents to automated systems, we provide solutions tailored to your analytical needs. Contact us today to discover how we can help you achieve faster, more reliable results.
Visual Guide
Related Products
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
- Three-dimensional electromagnetic sieving instrument
- Laboratory Test Sieves and Sieving Machines
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Laboratory Single Horizontal Jar Mill
People Also Ask
- How do you measure thin film SEM thickness? A Direct Visual Guide for Accurate Analysis
- What are the five methods used to sterilize materials in a laboratory? A Guide to Matching Method to Material
- How long does XRF analysis take? From Seconds to Hours, A Guide to Accurate Results
- How does a high-precision temperature control heating system ensure accurate corrosion kinetics? Expert Lab Solutions
- What is the key difference between calcination and sintering? Understanding Purification vs. Consolidation
- How does Hot Isostatic Pressing (HIP) optimize additive manufactured Inconel 718? Achieve 100% Density and Integrity
- At what temperature does THC distillate degrade? A Guide to Preserving Potency and Purity
- How does a laboratory forced-air drying oven process ternary nanocomposite products? Ensure Nanostructural Integrity


















