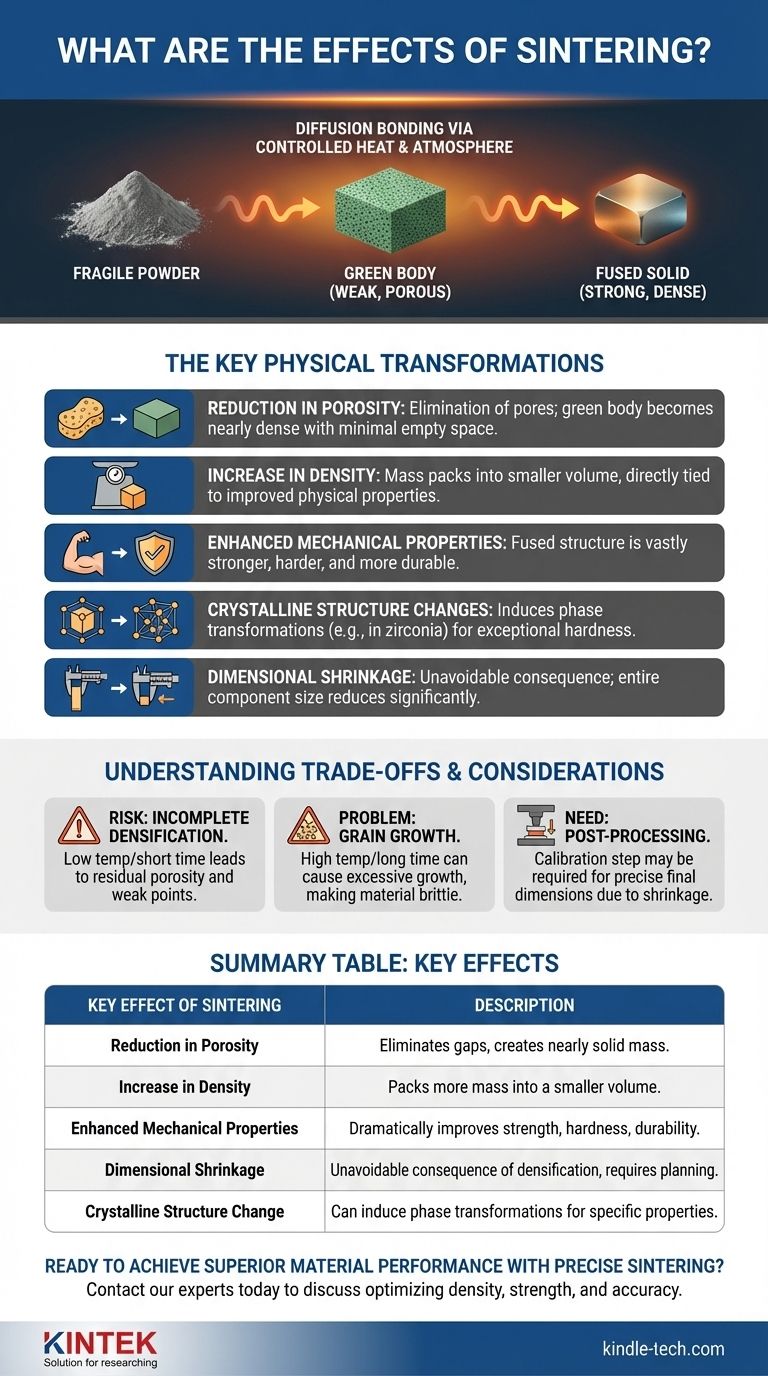
At its core, sintering transforms a fragile, compacted powder into a dense, strong, solid object. This is achieved by heating the material below its melting point, causing individual particles to fuse together through a process called diffusion bonding. The primary effects are a significant reduction in porosity, an increase in density, and a dramatic improvement in mechanical properties like strength and hardness.
The fundamental effect of sintering is the elimination of empty space. By using heat to bond particles at a molecular level, the process removes the pores between them, fundamentally altering the material's microstructure from a loose collection of grains into a solid, cohesive mass.

From Fragile "Green" Part to Fused Solid
Sintering is not a single event but a controlled thermal process that systematically evolves a material's internal structure. It begins with a weakly bound part and ends with a robust, functional component.
The Starting Point: The Green Body
Before sintering, the material exists as a "green body" or "green piece." This is a component formed by pressing a powder mixture into a desired shape using a mold and die.
This green body has the correct geometry but is porous and mechanically weak, easily crumbled or broken.
The Role of Heat and Atmosphere
The green body is placed in a furnace with a controlled atmosphere to prevent oxidation or other unwanted chemical reactions.
It is then heated to a high temperature that is critically below the material's melting point. This is a key distinction; the material does not become liquid.
Binder Burn-Off
In the initial heating stages, any residual organic binders used to help form the green body are cleanly burned off, leaving behind only the primary material particles.
The Mechanism of Diffusion Bonding
At elevated temperatures, atoms become highly active and begin to migrate between the surfaces of particles at their points of contact. This atomic movement is called solid-state diffusion.
This diffusion causes necks to form and grow between adjacent particles, effectively welding them together on a microscopic scale.
Particle Rearrangement and Densification
As these bonds grow, they create forces that pull the centers of the particles closer together. This collective movement causes particles to rearrange and pack more tightly.
The direct result is that the gaps, or pores, between the particles begin to shrink and eventually disappear.
The Key Physical Transformations
The microscopic process of diffusion bonding creates several critical macroscopic effects, which are the ultimate goals of sintering.
Reduction in Porosity
The most significant effect is the elimination of pores. A green body can have high porosity, but a fully sintered part will be nearly or completely dense, with very little empty space remaining.
Increase in Density
As porosity decreases, the material's density naturally increases. More mass is packed into a smaller volume, which is directly tied to the improvement of other physical properties.
Enhanced Mechanical Properties
The fused, dense microstructure is vastly stronger and more resistant to fracture than the initial powder compact. Sintering is directly responsible for a material's final strength, hardness, and durability.
Crystalline Structure Changes
For certain materials, such as zirconia used in dental ceramics, sintering induces a phase transformation. It changes the material's crystalline structure (e.g., from monoclinic to tetragonal), which is what gives the final part its exceptional hardness.
Dimensional Shrinkage
A direct and unavoidable consequence of eliminating pores is that the entire component shrinks in size. This shrinkage must be precisely calculated and accounted for during the initial mold design to achieve accurate final dimensions.
Understanding the Trade-offs and Considerations
While powerful, sintering is a precision process where control is paramount. Failing to manage the variables can lead to undesirable outcomes.
The Risk of Incomplete Densification
If the temperature is too low or the time is too short, sintering will be incomplete. This leaves residual porosity in the final part, creating weak points that compromise its mechanical integrity.
The Problem of Grain Growth
If the temperature is too high or held for too long, excessive grain growth can occur. While particles fuse, individual crystal grains can grow too large, which can sometimes make the material more brittle.
The Need for Post-Processing
Because shrinkage can be difficult to predict perfectly, parts requiring very tight tolerances may need a secondary calibration or sizing step after sintering. This involves pressing the part again in a precision die to correct minor dimensional deviations.
Liquid Phase Sintering
In some processes, a small amount of an additive is used that will melt at the sintering temperature. This "liquid phase" can flow into the remaining pores, accelerating the densification process and helping to achieve a fully dense final part.
Applying This to Your Goal
The effects of sintering are harnessed to achieve specific engineering outcomes. Your focus will determine which effect is most critical to monitor and control.
- If your primary focus is mechanical strength: The key effect is the diffusion bonding of particles, which creates a monolithic structure that is far stronger than the original powder compact.
- If your primary focus is achieving high density: Sintering accomplishes this by pulling particles together, systematically eliminating the pores that exist between them in the green state.
- If your primary focus is dimensional accuracy: You must account for the significant and unavoidable shrinkage that occurs as the part densifies during the process.
- If your primary focus is specific material properties (e.g., hardness in zirconia): Sintering can be used to induce critical phase transformations in the material's crystalline structure.
Ultimately, sintering is the essential process that converts a shaped collection of individual particles into a unified, functional, and robust component.
Summary Table:
| Key Effect of Sintering | Description |
|---|---|
| Reduction in Porosity | Eliminates gaps between particles, creating a nearly solid mass. |
| Increase in Density | Packs more mass into a smaller volume as pores are removed. |
| Enhanced Mechanical Properties | Dramatically improves final strength, hardness, and durability. |
| Dimensional Shrinkage | An unavoidable consequence of densification that must be calculated in advance. |
| Crystalline Structure Change | Can induce phase transformations (e.g., in zirconia) for specific properties. |
Ready to achieve superior material performance with precise sintering?
KINTEK specializes in providing the high-quality lab furnaces and consumables you need for controlled thermal processes. Whether you are developing stronger metal components, advanced ceramics, or other powder-based materials, our equipment ensures the precise temperature and atmosphere control critical for successful sintering outcomes.
Contact our experts today to discuss how our solutions can help you optimize density, strength, and dimensional accuracy in your sintered products.
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Spark Plasma Sintering Furnace SPS Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
People Also Ask
- Why is sintering easier in the presence of a liquid phase? Unlock Faster, Lower-Temperature Densification
- Why is a high vacuum required for sintering Ti-43Al-4Nb-1Mo-0.1B? Ensure Purity & Fracture Toughness
- How does a high-temperature vacuum sintering furnace facilitate the post-treatment of Zirconia coatings?
- Why must green bodies produced via binder jetting undergo treatment in a vacuum sintering furnace?
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost



















